Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
The use of ketogenic diet in therapy of drug-resistant epilepsies – current state of knowledge
1
Faculty of Medicine, Medical University of Silesia, Zabrze, Poland
2
Medical University, Wroclaw, Poland
Corresponding author
J Pre Clin Clin Res. 2023;17(3):157-166
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Epilepsy is a neurological disorder where a desynchronization occurs in the discharge of neurons of specific brain areas. Clinically, this manifests as epileptic seizures with possible disturbances of consciousness. Therapy of epilepsy is based on pharmacotherapy, but in cases of drug resistance, alternative methods, such as ketogenic diet (KD) should be explored. The aim of this review is to present the current state of knowledge concerning the use of KD in epilepsy and the clinical results of such therapy.
Review methods:
PubMed, PubMed Central and Google Scholar databases were searched using key words related to epilepsy, ketogenic diet, metabolic mechanisms of ketosis and antiepileptic effects of ketone bodies. Articles and book sections in English were searched and reviewed. Articles were selected after analyzing abstracts and those that matched and described the topic in a proper way were used. The types of articles included prospective studies, retrospective studies, reviews and meta-analyses.
Brief description of the state of knowledge:
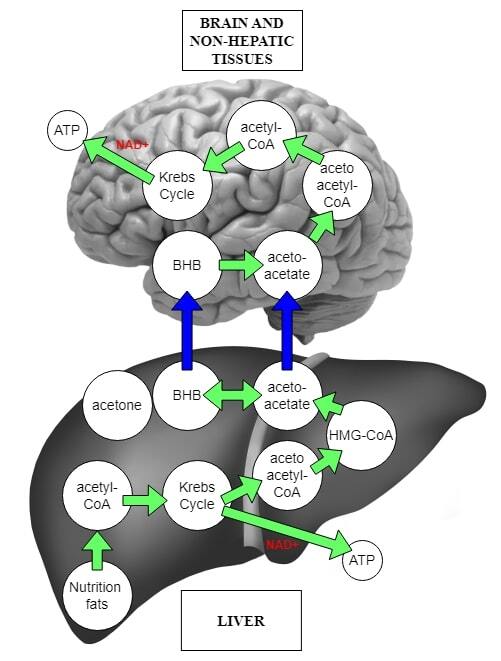
From the initial search, 20 articles strictly concerning the KD mechanisms of action, its antiepileptic effects and the results of the therapy conducted were retrieved for final analysis. Available data provide the information specifically on the definition of KD, adverse effects, anti-seizure mechanisms and therapy results mainly from recent years. KD is currently being used as an alternative therapy in drug-resistant epilepsies. The reduction in seizures after its use averages about 50%, and some studies have shown up to 90% effectiveness in seizure reduction.
Summary:
Understanding the mechanisms of brain metabolism allowed the use of KD in the treatment of epilepsy and other neuropsychiatric diseases. The results of the therapy appear to be satisfactory and provide hope for future epilepsy therapy.
Epilepsy is a neurological disorder where a desynchronization occurs in the discharge of neurons of specific brain areas. Clinically, this manifests as epileptic seizures with possible disturbances of consciousness. Therapy of epilepsy is based on pharmacotherapy, but in cases of drug resistance, alternative methods, such as ketogenic diet (KD) should be explored. The aim of this review is to present the current state of knowledge concerning the use of KD in epilepsy and the clinical results of such therapy.
Review methods:
PubMed, PubMed Central and Google Scholar databases were searched using key words related to epilepsy, ketogenic diet, metabolic mechanisms of ketosis and antiepileptic effects of ketone bodies. Articles and book sections in English were searched and reviewed. Articles were selected after analyzing abstracts and those that matched and described the topic in a proper way were used. The types of articles included prospective studies, retrospective studies, reviews and meta-analyses.
Brief description of the state of knowledge:
From the initial search, 20 articles strictly concerning the KD mechanisms of action, its antiepileptic effects and the results of the therapy conducted were retrieved for final analysis. Available data provide the information specifically on the definition of KD, adverse effects, anti-seizure mechanisms and therapy results mainly from recent years. KD is currently being used as an alternative therapy in drug-resistant epilepsies. The reduction in seizures after its use averages about 50%, and some studies have shown up to 90% effectiveness in seizure reduction.
Summary:
Understanding the mechanisms of brain metabolism allowed the use of KD in the treatment of epilepsy and other neuropsychiatric diseases. The results of the therapy appear to be satisfactory and provide hope for future epilepsy therapy.
Jezierzański MM, Furgoł T, Miciak M. The use of ketogenic diet in therapy of drug-resistant epilepsies – current state of knowledge. J Pre-Clin Clin Res. 2023; 17(3): 157–166. doi: 10.26444/jpccr/168678
REFERENCES (90)
1.
Saxena S, Li S. Defeating epilepsy: A global public health commitment. Epilepsia Open. 2017;2:153–55. https://doi.org/10.1002/epi4.1....
2.
Megiddo I, Colson A, Chisholm D, et al. Health and economic benefits of public financing of epilepsy treatment in India: An agent-based simulation model. Epilepsia. 2016;57:464–74. https://doi.org/10.1111/epi.13....
3.
Beghi E. The Epidemiology of Epilepsy. Neuroepidemiology. 2020;54:185–191. https://doi.org/10.1159/000503....
4.
Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49:8–12. https://doi.org/10.1111/j.1528....
5.
Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303. https://doi.org/10.1212/WNL.00....
6.
Camfield P, Camfield C. Incidence, prevalence and aetiology of seizures and epilepsy in children. Epileptic Disord. 2015;17:117–23. https://doi.org/10.1684/epd.20....
7.
WHO. Global burden of epilepsy and the need for coordinated action at the country level to address its health, social and public knowledge implications. Executive Board 136. 2015.
8.
Scheffer IE, Berkovic S, Capovilla G, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–21. https://doi.org/10.1111/epi.13....
9.
Aaberg KM, Surén P, Søraas CL, et al. Seizures, syndromes, and etiologies in childhood epilepsy: The International League Against Epilepsy 1981, 1989, and 2017 classifications used in a population-based cohort. Epilepsia. 2017;58:1880–1891. https://doi.org/10.1111/epi.13....
10.
Liu S, YuW, Lü Y. The causes of new-onset epilepsy and seizures in the elderly. Neuropsychiatr Dis Treat. 2016;12:1425–1434. https://doi.org/10.2147/NDT.S1....
11.
Bosak M, Słowik A, Kacorzyk R, et al. Implementation of the new ILAE classification of epilepsies into clinical practice – a cohort study. Epilepsy Behav. 2019;96:28–32. https://doi.org/10.1016/j.yebe....
13.
Prayson RA. Pathology of Epilepsy. In: Perry A, Brat DJ, editors. Practical Surgical Neuropathology: A Diagnostic Approach. Elsevier; 2018. p. 617–632. https://doi.org/10.1016/b978-0....
14.
Stafstrom CE, Carmant L. Seizures and epilepsy: an overview for neuroscientists. Cold Spring Harb Perspect Med. 2015;5:a022426. https://doi.org/10.1101/cshper....
15.
Nashef L. Sudden unexpected death in epilepsy: terminology and definitions. Epilepsia. 1997;38:S6–S8. https://doi.org/10.1111/j.1528....
16.
Devinsky O, Hesdorffer DC, Thurman DJ, et al. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet Neurol. 2016;15:1075–1088. https://doi.org/10.1016/S1474-....
17.
Harden C, Tomson T, Gloss D, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2017;88:1674–1680. https://doi.org/10.1212/WNL.00....
18.
1Strzelczyk A, Schubert-Bast S. Expanding the Treatment Landscape for Lennox-Gastaut Syndrome: Current and Future Strategies. CNS Drugs. 2021;35:61–83. https://doi.org/10.1007/s40263....
19.
Jahngir MU, Ahmad MQ, Jahangir M. Lennox-Gastaut Syndrome: In a Nutshell. Cureus. 2018;10:e3134. https://doi.org/10.7759/cureus....
20.
Franzini A, Cordella R, Messina G, et al. Targeting the brain: considerations in 332 consecutive patients treated by deep brain stimulation (DBS) for severe neurological diseases. Neurol Sci. 2012;33:1285–1303. https://doi.org/10.1007/s10072....
21.
Pavone P, Polizzi A, Marino SD, et al. West syndrome: a comprehensive review. Neurol Sci. 2020;41:3547–3562. https://doi.org/10.1007/s10072....
22.
Guerrero Ruiz GDP. Encefalopatías epilépticas de inicio en recién nacidos y lactantes [Epileptic encephalopathies of onset in neonates and infants]. Medicina (B Aires). 2022;82:13–18.
23.
Ohtahara S, Yamatogi Y. Ohtahara syndrome: With special reference to its developmental aspects for differentiating from early myoclonic encephalopathy. Epilepsy Res. 2006;70:S58–S67. https://doi.org/10.1016/j.eple....
24.
Lee EH. Epilepsy syndromes during the first year of life and the usefulness of an epilepsy gene panel. Korean J Pediatr. 2018;61:101–107. https://doi.org/10.3345/kjp.20....
25.
Wolff M, Johannesen KM, Hedrich UBS, et al. Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain. 2017;140:1316–1336. https://doi.org/10.1093/brain/....
26.
Borlot F, Abushama A, Morrison-Levy N, et al. KCNT1-related epilepsy: An international multicenter cohort of 27 pediatric cases. Epilepsia. 2020;61:679–692. https://doi.org/10.1111/epi.16....
27.
Bayat A, Bayat M, Rubboli G, et al. Epilepsy Syndromes in the First Year of Life and Usefulness of Genetic Testing for Precision Therapy. Genes. 2021;12:1051. https://doi.org/10.3390/genes1....
29.
Anwar A, Saleem S, Patel UK, et al. Dravet Syndrome: An Overview. Cureus. 2019;11:e5006. doi: https://doi.org/10.7759/cureus....
30.
Samaitienė R, Norkūnienė J, Tumienė B, et al. Sleep and behavioral problems in rolandic epilepsy. Pediatr Neurol. 2013;48:115–122. https://doi.org/10.1016/j.pedi....
31.
Pohlmann-Eden B, Aldenkamp A, Baker GA, et al. The relevance of neuropsychiatric symptoms and cognitive problems in new-onset epilepsy – Current knowledge and understanding. Epilepsy Behav. 2015;51:199–209. https://doi.org/10.1016/j.yebe....
32.
Rejdak K, Rola R, Mazurkiewicz-Bełdzińska M, et al. Diagnostyka i leczenie padaczki u osób dorosłych—rekomendacje Polskiego Towarzystwa Neurologicznego. Pol Przegl Neurol. 2016;12:15–27.
33.
Abramovici S, Bagić A. Epidemiology of epilepsy. Handb Clin Neurol. 2016;138:159–171. https://doi.org/10.1016/B978-0....
34.
Behr C, Goltzene MA, Kosmalski G, et al. Epidemiology of epilepsy. Rev Neurol (Paris). 2016;172:27–36. https://doi.org/10.1016/j.neur....
35.
Perucca P, Scheffer IE, Kiley M. The management of epilepsy in children and adults. Med J Aust. 2018;208:226–233. https://doi.org/10.5694/mja17.....
36.
Perucca P, Jacoby A, Marson AG, et al. Adverse antiepileptic drug effects in new-onset seizures: a case-control study. Neurology. 2011;76:273–279. https://doi.org/10.1212/WNL.0b....
37.
Perucca E, Tomson T. The pharmacological treatment of epilepsy in adults. Lancet Neurol. 2011;10:446–456. https://doi.org/10.1016/S1474-....
38.
Brodie MJ, Barry SJ, Bamagous GA, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–1554. https://doi.org/10.1212/WNL.0b....
39.
Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–1015. https://doi.org/10.1016/S0140-....
40.
Brodie MJ, Perucca E, Ryvlin P, et al. Levetiracetam Monotherapy Study Group. Comparison of levetiracetam and controlled-release carbamazepine in newly diagnosed epilepsy. Neurology 2007;68:402–408. https://doi.org/10.1212/01.wnl....
41.
Baulac M, Brodie MJ, Patten A, et al. Efficacy and tolerability of zonisamide versus controlled-release carbamazepine for newly diagnosed partial epilepsy: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2012;11:579–588. https://doi.org/10.1016/S1474-....
42.
Baulac M, Rosenow F, Toledo M, et al. Efficacy, safety, and tolerability of lacosamide monotherapy versus controlled-release carbamazepine in patients with newlydiagnosed epilepsy: a phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2017;16:43–54. https://doi.org/10.1016/S1474-....
43.
Tomson T, Marson A, Boon P, et al. Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia. 2015;56:1006–1019. https://doi.org/10.1111/epi.13....
44.
Patel SI, Pennell PB. Management of epilepsy during pregnancy: an update. Ther Adv Neurol Disord. 2016;9:118–129. https://doi.org/10.1177/175628....
45.
Ryvlin P, Cross JH, Rheims S. Epilepsy surgery in children and adults. Lancet Neurol. 2014;13:1114–1126. https://doi.org/10.1016/S1474-....
46.
Ramos-Perdigués S, Baillés E, Mané A, et al. A prospective study contrasting the psychiatric outcome in drug-resistant epilepsy between patients who underwent surgery and a control group. Epilepsia. 2016;57:1680–1690. https://doi.org/10.1111/epi.13....
47.
Englot DJ, Ouyang D, Garcia PA, et al. Epilepsy surgery trends in the United States, 1990–2008. Neurology. 2012;78:1200–1206. https://doi.org/10.1212/WNL.0b....
48.
Boison D. New insights into the mechanisms of the ketogenic diet. Curr Opin Neurol. 2017;30:187–192. https://doi.org/10.1097/WCO.00....
49.
Ułamek-Kozioł M, Czuczwar SJ, Januszewski S, et al. Ketogenic Diet and Epilepsy. Nutrients. 2019;11:2510. https://doi.org/10.3390/nu1110....
50.
D’Andrea Meira I, Romão TT, Pires do Prado HJ, et al. Ketogenic Diet and Epilepsy: What We Know So Far. Front Neurosci. 2019;13:5. https://doi.org/10.3389/fnins.....
51.
Sampaio LP. Ketogenic diet for epilepsy treatment. Arq Neuropsiquiatr. 2016;74:842–848. https://doi.org/10.1590/0004-2....
52.
Rezaei S, Abdurahman AA, Saghazadeh A, et al. Short-term and long-term efficacy of classical ketogenic diet and modified Atkins diet in children and adolescents with epilepsy: A systematic review and meta-analysis. Nutr Neurosci. 2019;22:317–334. https://doi.org/10.1080/102841....
53.
Barzegar M, Afghan M, Tarmahi V, et al. Ketogenic diet: overview, types, and possible anti-seizure mechanisms. Nutr Neurosci. 2021;24:307–316. https://doi.org/10.1080/102841....
54.
Rudy L, Carmen R, Daniel R, et al. Anticonvulsant mechanisms of the ketogenic diet and caloric restriction. Epilepsy Res. 2020;168:106499. https://doi.org/10.1016/j.eple....
55.
Han FY, Conboy-Schmidt L, Rybachuk G, et al. Dietary medium chain triglycerides for management of epilepsy: New data from human, dog, and rodent studies. Epilepsia. 2021;62:1790–1806. https://doi.org/10.1111/epi.16....
56.
Prasoppokakorn T, Jirasakuldej S, Lakananurak N. Medium-chain triglyceride ketogenic diet is effective for treatment of an adult with super-refractory status epilepticus: a case report and literature review. Eur J Clin Nutr. 2019;73:1594–1597. https://doi.org/10.1038/s41430....
57.
Liu YM, Wang HS. Medium-chain triglyceride ketogenic diet, an effective treatment for drug-resistant epilepsy and a comparison with other ketogenic diets. Biomed J. 2013;36:9–15. https://doi.org/10.4103/2319-4....
58.
Rezaei S, Harsini S, Kavoosi M, et al. Efficacy of low glycemic index treatment in epileptic patients: a systematic review. Acta Neurol Belg. 2018;118:339–349. https://doi.org/10.1007/s13760....
59.
Murakami M, Tognini P. Molecular Mechanisms Underlying the Bioactive Properties of a Ketogenic Diet. Nutrients. 2022;14:782. https://doi.org/10.3390/nu1404....
60.
Luong TV, Abild CB, Bangshaab M, et al. Ketogenic Diet and Cardiac Substrate Metabolism. Nutrients. 2022;14:1322. https://doi.org/10.3390/nu1407....
61.
Choi YJ, Jeon SM, Shin S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients. 2020;12:2005. https://doi.org/10.3390/nu1207....
62.
Dąbek A, Wojtala M, Pirola L, et al. Modulation of Cellular Biochemistry, Epigenetics and Metabolomics by Ketone Bodies. Implications of the Ketogenic Diet in the Physiology of the Organism and Pathological States. Nutrients. 2020;12:788. https://doi.org/10.3390/nu1203....
63.
Azevedo de Lima P, Baldini Prudêncio M, Murakami DK, et al. Effect of classic ketogenic diet treatment on lipoprotein subfractions in children and adolescents with refractory epilepsy. Nutrition. 2017;33:271–277. https://doi.org/10.1016/j.nut.....
64.
Zhu H, Bi D, Zhang Y, et al. Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations. Signal Transduct Target Ther. 2022;7:11. https://doi.org/10.1038/s41392....
65.
Sondhi V, Agarwala A, Pandey RM, et al. Efficacy of Ketogenic Diet, Modified Atkins Diet, and Low Glycemic Index Therapy Diet Among Children With Drug-Resistant Epilepsy: A Randomized Clinical Trial. JAMA Pediatr. 2020;174:944–951. https://doi.org/10.1001/jamape....
66.
Pizzo F, Collotta AD, Di Nora A, et al. Ketogenic diet in pediatric seizures: a randomized controlled trial review and meta-analysis. Expert Rev Neurother. 2022;22:169–177. https://doi.org/10.1080/147371....
67.
Lyons L, Schoeler NE, Langan D, et al. Use of ketogenic diet therapy in infants with epilepsy: A systematic review and meta-analysis. Epilepsia. 2020;61:1261–1281. https://doi.org/10.1111/epi.16....
68.
Wang YQ, Fang ZX, Zhang YW, et al. Efficacy of the ketogenic diet in patients with Dravet syndrome: A meta-analysis. Seizure. 2020;81:36–42. https://doi.org/10.1016/j.seiz....
69.
Dou X, Xu X, Mo T, et al. Evaluation of the seizure control and the tolerability of ketogenic diet in Chinese children with structural drug-resistant epilepsy. Seizure. 2022;94:43–51. https://doi.org/10.1016/j.seiz....
70.
Green SF, Nguyen P, Kaalund-Hansen K, et al. Effectiveness, retention, and safety of modified ketogenic diet in adults with epilepsy at a tertiary-care centre in the UK. J Neurol. 2020;267:1171–1178. https://doi.org/10.1007/s00415....
71.
Roehl K, Falco-Walter J, Ouyang B, et al. Modified ketogenic diets in adults with refractory epilepsy: Efficacious improvements in seizure frequency, seizure severity, and quality of life. Epilepsy Behav. 2019;93:113–118. https://doi.org/10.1016/j.yebe....
72.
Husari KS, Cervenka MC. The ketogenic diet all grown up-Ketogenic diet therapies for adults. Epilepsy Res. 2020;162:106319. https://doi.org/10.1016/j.eple....
73.
Lotankar S, Prabhavalkar KS, Bhatt LK. Biomarkers for Parkinson’s Disease: Recent Advancement. Neurosci Bull. 2017;33:585–597. https://doi.org/10.1007/s12264....
74.
Caputi V, Giron MC. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int J Mol Sci. 2018;19:1689. https://doi.org/10.3390/ijms19....
75.
Chia SJ, Tan EK, Chao YX. Historical Perspective: Models of Parkinson’s Disease. Int J Mol Sci. 2020;21:2464. https://doi.org/10.3390/ijms21....
76.
Apostolova LG. Alzheimer Disease. Continuum (Minneap Minn). 2016;22:419–434. https://doi.org/10.1212/CON.00....
77.
Klimova B, Novotný M, Kuca K, et al. Effect Of An Extra-Virgin Olive Oil Intake On The Delay Of Cognitive Decline: Role Of Secoiridoid Oleuropein?. Neuropsychiatr Dis Treat. 2019;15:3033–3040. https://doi.org/10.2147/NDT.S2....
78.
Albury CL, Stuart S, Haupt LM, et al. Ion channelopathies and migraine pathogenesis. Mol Genet Genomics. 2017;292:729–739. https://doi.org/10.1007/s00438....
79.
Hsiao FJ, Chen WT, Pan LH, et al. Dynamic brainstem and somatosensory cortical excitability during migraine cycles. J Headache Pain. 2022;23:21. https://doi.org/10.1186/s10194....
80.
de Almeida Rabello Oliveira M, da Rocha Ataíde T, de Oliveira SL, et al. Effects of short-term and long-term treatment with medium- and long-chain triglycerides ketogenic diet on cortical spreading depression in young rats. Neurosci Lett. 2008;434:66–70. https://doi.org/10.1016/j.neul....
81.
Włodarczyk A, Cubała WJ, Stawicki M. Ketogenic diet for depression: A potential dietary regimen to maintain euthymia?. Prog Neuropsychopharmacol Biol Psychiatry. 2021;109:110257. https://doi.org/10.1016/j.pnpb....
82.
Shimazu T, Hirschey MD, Newman J, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. https://doi.org/10.1126/scienc....
83.
Norgren J, Daniilidou M, Kåreholt I, et al. Serum proBDNF Is Associated With Changes in the Ketone Body β-Hydroxybutyrate and Shows Superior Repeatability Over Mature BDNF: Secondary Outcomes From a Cross-Over Trial in Healthy Older Adults. Front Aging Neurosci. 2021;13:716594. https://doi.org/10.3389/fnagi.....
84.
Tillery EE, Ellis KD, Threatt TB, et al. The use of the ketogenic diet in the treatment of psychiatric disorders. Ment Health Clin. 2021;11:211–219. https://doi.org/10.9740/mhc.20....
85.
van der Louw E, van den Hurk D, Neal E, et al. Eur J Paediatr Neurol. 2016;20:798–809. https://doi.org/10.1016/j.ejpn....
86.
Schoeler NE, Cross JH. Ketogenic dietary therapies in adults with epilepsy: a practical guide. Pract Neurol. 2016;16:208–214. https://doi.org/10.1136/practn....
87.
Wells J, Swaminathan A, Paseka J, et al. Efficacy and Safety of a Ketogenic Diet in Children and Adolescents with Refractory Epilepsy-A Review. Nutrients. 2020;12:1809. https://doi.org/10.3390/nu1206....
88.
Svedlund A, Hallböök T, Magnusson P, et al. Prospective study of growth and bone mass in Swedish children treated with the modified Atkins diet. Eur J Paediatr Neurol. 2019;23:629–638. https://doi.org/10.1016/j.ejpn....
89.
Armeno M, Verini A, Del Pino M, et al. A Prospective Study on Changes in Nutritional Status and Growth Following Two Years of Ketogenic Diet (KD) Therapy in Children with Refractory Epilepsy. Nutrients. 2019;11:1596. https://doi.org/10.3390/nu1107....
90.
Ferraris C, Guglielmetti M, Pasca L, et al. Impact of the Ketogenic Diet on Linear Growth in Children: A Single-Center Retrospective Analysis of 34 Cases. Nutrients. 2019;11:1442. https://doi.org/10.3390/nu1107....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.