Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Immunopathogenesis of multiple sclerosis – mechanisms of autoimmunity,
neuroinflammation and strategies of treatment
1
Student Research Group, Department and Clinic of Neurology, University Clinical Hospital No. 4, Medical University, Lublin, Poland
2
Student Scientific Association, Department of Pneumonology, Allergology, and Oncology, Medical University, Lublin, Poland
3
Department and Clinic of Neurology, University Clinical Hospital No. 4, Medical University, Lublin, Poland
Corresponding author
Filip Kazimierz Gajewski
Student Scientific Association at the Department of Pneumonology, Allergology, and Oncolog, Medical University, Doktora Kazimierza Jaczewskiego 8, 20-090 Lublin, Poland
Student Scientific Association at the Department of Pneumonology, Allergology, and Oncolog, Medical University, Doktora Kazimierza Jaczewskiego 8, 20-090 Lublin, Poland
KEYWORDS
multiple sclerosisimmunopathogenesisTh17 cellsregulatory T cells (Treg)B lymphocytesblood–brain barrierdemyelinationmicrogliadisease-modifying therapiesImmune tolerance / tolerogenic vaccines
TOPICS
ABSTRACT
Introduction and objective:
The aim of the review is to summarize current understanding of multiple sclerosis (MS) immunopathogenesis, focusing on Th17 cells, regulatory T cells, B lymphocytes, and innate immune components, while outlining contemporary and emerging therapeutic strategies
Review methods:
A literature search was conducted in PubMed and Scopus using the key words ‘multiple sclerosis’, ‘immunotherapy’, ‘autoimmunity’, ‘Th17 Cells’, and ‘B-Lymphocytes’ for publications occurring in publications between 2019–2025. A total of 412,560 articles were found – 173,450 were from PubMed and 239,110 from Scopus. After applying inclusion criteria to original research, reviews, book chapters, and editorials in English, 49 articles were selected for a narrative review. Over 96% of included studies were published within the last three years, reflecting a rapidly growing interest in the topic.
Brief description of the state of knowledge:
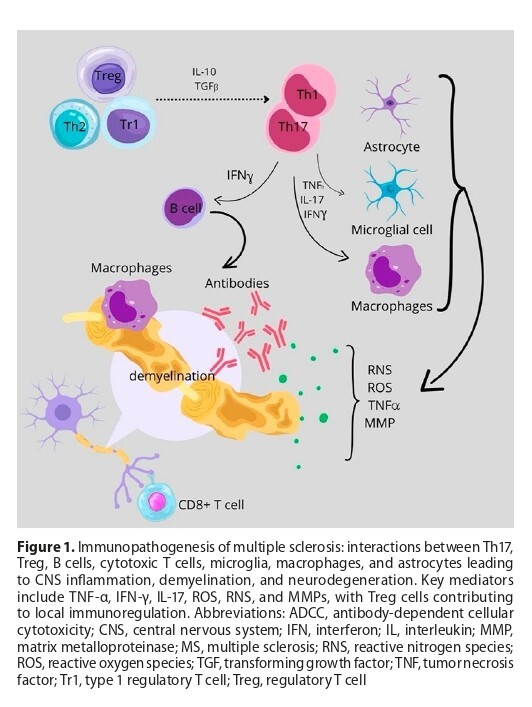
MS results from complex interactions among Th17 cells, regulatory T cells, B cells, microglia, macrophages, and astrocytes, leading to blood–brain barrier disruption, demyelination, axonal injury, and neurodegeneration. Therapeutic strategies have evolved from interferons and glatiramer acetate to oral agents, sphingosine-1-phosphate receptor modulators, monoclonal antibodies, and B-cell depleting therapies, enhancing disease control. Emerging approaches, including haematopoietic stem cell transplantation and peptide- or nanovaccine-based therapies, aim to restore immune tolerance with minimal systemic immunosuppression.
Summary:
MS arises from an imbalance between pro-inflammatory and regulatory immune mechanisms. Insights into these pathways have provided information about the development of targeted, individualized treatments. Further research into immune modulation and neuroprotection may enable durable remission, prevent neurodegeneration, and improve patient outcomes.
The aim of the review is to summarize current understanding of multiple sclerosis (MS) immunopathogenesis, focusing on Th17 cells, regulatory T cells, B lymphocytes, and innate immune components, while outlining contemporary and emerging therapeutic strategies
Review methods:
A literature search was conducted in PubMed and Scopus using the key words ‘multiple sclerosis’, ‘immunotherapy’, ‘autoimmunity’, ‘Th17 Cells’, and ‘B-Lymphocytes’ for publications occurring in publications between 2019–2025. A total of 412,560 articles were found – 173,450 were from PubMed and 239,110 from Scopus. After applying inclusion criteria to original research, reviews, book chapters, and editorials in English, 49 articles were selected for a narrative review. Over 96% of included studies were published within the last three years, reflecting a rapidly growing interest in the topic.
Brief description of the state of knowledge:
MS results from complex interactions among Th17 cells, regulatory T cells, B cells, microglia, macrophages, and astrocytes, leading to blood–brain barrier disruption, demyelination, axonal injury, and neurodegeneration. Therapeutic strategies have evolved from interferons and glatiramer acetate to oral agents, sphingosine-1-phosphate receptor modulators, monoclonal antibodies, and B-cell depleting therapies, enhancing disease control. Emerging approaches, including haematopoietic stem cell transplantation and peptide- or nanovaccine-based therapies, aim to restore immune tolerance with minimal systemic immunosuppression.
Summary:
MS arises from an imbalance between pro-inflammatory and regulatory immune mechanisms. Insights into these pathways have provided information about the development of targeted, individualized treatments. Further research into immune modulation and neuroprotection may enable durable remission, prevent neurodegeneration, and improve patient outcomes.
REFERENCES (48)
1.
Schirmer L, Velmeshev D, Holmqvist S, et al. Neuronal vulnerability and multilineage diversity in multiple sclerosis. Nature. 2019;573(7772):75–82. doi:10.1038/s41586-019-1404-z.
2.
Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816–1821. doi:10.1177/1352458520970841.
3.
Rzepińska M, Rzepiński Ł, Steinborn B. Multiple sclerosis in women – selected epidemiological, clinical, therapeutic and maternal aspects. Current Neurology. 2022;22(1):25–31. doi:10.15557/AN.2022.0004.
4.
Brola W, Steinborn B. Pediatric multiple sclerosis – current status of epidemiology, diagnosis and treatment. Neurol Neurochir Pol. 2020;54(6):508–517. doi:10.5603/PJNNS.a2020.0069.
5.
Filippi M, Preziosa P, Barkhof F, et al. Diagnosis of progressive multiple sclerosis from the imaging perspective: a review. JAMA Neurol. 2021;78(3):351–364. doi:10.1001/jamaneurol.2020.4689.
6.
Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol. 2019;26(1):27–40. doi:10.1111/ene.13819.
7.
Zarobkiewicz MK, Morawska I, Michalski A, et al. NKT and NKTlike cells in autoimmune neuroinflammatory diseases—multiple sclerosis, myasthenia gravis and Guillain-Barre syndrome. Int J Mol Sci. 2021;22(17):9520. doi:10.3390/ijms22179520.
8.
Rodríguez Murúa S, Farez MF, Quintana FJ. The immune response in multiple sclerosis. Annu Rev Pathol. 2022;17:121–139. doi:10.1146/annurev-pathol-052920-040318.
9.
Arneth BM. Impact of B cells to the pathophysiology of multiple sclerosis. J Neuroinflammation. 2019;16(1):128. doi:10.1186/s12974-019-1517-1.
10.
Moser T, Akgün K, Proschmann U, et al. The role of TH17 cells in multiple sclerosis: Therapeutic implications. Autoimmun Rev. 2020;19(10):102647. doi:10.1016/j.autrev.2020.102647.
11.
Vasileiou ES, Fitzgerald KC. Multiple sclerosis pathogenesis and updates in targeted therapeutic approaches. Curr Allergy Asthma Rep. 2023;23:481–496. doi:10.1007/s11882-023-01102-0.
12.
Martinsen V, Kursula P. Multiple sclerosis and myelin basic protein: insights into protein disorder and disease. Amino Acids. 2022;54(1):99–109. doi:10.1007/s00726-021-03111-7.
13.
Liu R, Du S, Zhao L, et al. Autoreactive lymphocytes in multiple sclerosis: Pathogenesis and treatment target. Front Immunol. 2022;13:996469. doi:10.3389/fimmu.2022.996469.
14.
Haase S, Linker RA. Inflammation in multiple sclerosis. Ther Adv Neurol Disord. 2021;14:17562864211007687. doi:10.1177/17562864211007687.
15.
Salles D, Samartini RS, Alves MTS, et al. Functions of astrocytes in multiple sclerosis: a review. Mult Scler Relat Disord. 2022;60:103749. doi:10.1016/j.msard.2022.103749.
16.
Kuhn S, Gritti L, Crooks D, Dombrowski Y. Oligodendrocytes in development, myelin generation and beyond. Cells. 2019;8(11):1424. doi:10.3390/cells8111424.
17.
Jäkel S, Agirre E, Mendanha Falcão A, et al. Altered human oligodendrocyte heterogeneity in multiple sclerosis. Nature.2019;566(7745):543–547. doi:10.1038/s41586-019-0903-2.
18.
Calahorra L, Camacho-Toledano C, Serrano-Regal MP, et al. Regulatory cells in multiple sclerosis: from blood to brain. Biomedicines. 2022;10(2):335. doi:10.3390/biomedicines10020335.
19.
Khosravi M, Majdinasab N, Amari A, Ghadiri AA. Increased frequency of CD4+CD25high CD127low/- regulatory T cells in patients with multiple sclerosis. Gene Rep. 2019;17:100456. doi:10.1016/j. genrep.2019.100456.
20.
Verma ND, Lam AD, Chiu C, et al. Multiple sclerosis patients have reduced resting and increased activated CD4+CD25+FOXP3+T regulatory cells. Sci Rep. 2021;11(1):10476. doi:10.1038/s41598-021- 88448-5.
21.
Zarobkiewicz MK, Kowalska W, Roliński J, Bojarska-Junak AA. γδ T lymphocytes in the pathogenesis of multiple sclerosis and experimental autoimmune encephalomyelitis. J Neuroimmunol. 2019;330:67–73. doi:10.1016/j.jneuroim.2019.02.009.
22.
Holloman JP, Axtell RC, Monson NL, Wu GF. The role of B cells in primary progressive multiple sclerosis. Front Neurol. 2021;12:680581. doi:10.3389/fneur.2021.680581.
23.
DiSano KD, Gilli F, Pachner AR. Memory B cells in multiple sclerosis: emerging players in disease pathogenesis. Front Immunol. 2021;12:676686. doi:10.3389/fimmu.2021.676686.
24.
Carta S, Ferraro D, Ferrari S, et al. Oligoclonal bands: clinical utility and interpretation cues. Crit Rev Clin Lab Sci. 2022;59(6):391–404. doi:10.1080/10408363.2022.2039591.
25.
Kanatas P, Stouras I, Stefanis L, Stathopoulos P. B-cell-directed therapies: a new era in multiple sclerosis treatment. Can J Neurol Sci. 2023;50(3):355–364. doi:10.1017/cjn.2022.60.
26.
Bell L, Lenhart A, Rosenwald A, Monoranu CM, Berberich-Siebelt F. Lymphoid aggregates in the CNS of progressive multiple sclerosis patients lack regulatory T cells. Front Immunol. 2020;10:3090. doi:10.3389/fimmu.2019.03090.
27.
van Olst L, Rodriguez-Mogeda C, Picon C, et al. Meningeal inflammation in multiple sclerosis induces phenotypic changes in cortical microglia that differentially associate with neurodegeneration. Acta Neuropathol. 2021;141(6):881–899. doi:10.1007s00401-021-02293-4.
28.
Rommer PS, Milo R, Han MH, et al. Immunological aspects of approved MS therapeutics. Front Immunol. 2019;10:1564. doi:10.3389/fimmu.2019.01564.
29.
Bou Rjeily N, Mowry EM, Ontaneda D, Carlson AK. Highly effective therapy versus escalation approaches in early multiple sclerosis: what is the future of multiple sclerosis treatment? Neurol Clin. 2024;42(1):185–201. doi:10.1016/j.ncl.2023.06.004.
30.
Hauser SL, Cree BAC. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12):1380–1390.e2. doi:10.1016j.amjmed.2020.05.049.
31.
Arrambide G, Iacobaeus E, Amato MP, et al. Aggressive multiple sclerosis (2): treatment. Mult Scler J. 2020;26(9):1045–1063. doi:10.1177/1352458520924595.
32.
Gonzalez-Lorenzo M, Ridley B, Minozzi S, et al. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database Syst Rev. 2024;1(1):CD011381. doi:10.1002/14651858.CD011381.pub3.
33.
Okuda DT, Kantarci O, Lebrun-Frénay C, et al. Dimethyl fumarate delays multiple sclerosis in radiologically isolated syndrome. Ann Neurol. 2023;93(3):604–614. doi:10.1002/ana.26555.
34.
Klotz L, Havla J, Schwab N, et al. Risks and risk management in modern multiple sclerosis immunotherapeutic treatment. Ther Adv Neurol Disord. 2019;12:1756286419836571. doi:10.1177/1756286419836571.
35.
Lambrianides S, Kinnis E. Progressive multifocal leukoencephalopathy. Arch Hellenic Med. 2019;36:464–474.
36.
Zaffaroni M. Fingolimod in pediatric-onset multiple sclerosis. Neurol Sci. 2021;42(1):1–4. doi:10.1007/s10072-021-05294-z.
37.
Rojas JI, Patrucco L, Pappolla A, Sánchez F, Cristiano E. Brain volume loss and physical and cognitive impairment in naïve multiple sclerosis patients treated with fingolimod: prospective cohort study in Buenos Aires, Argentina. Arq Neuropsiquiatr. 2022;80(7):699–705. doi:10.1055/s-0042-1755277.
38.
Rammohan K, Coyle PK, Sylvester E, et al. The development of cladribine tablets for the treatment of multiple sclerosis: a comprehensive review. Drugs. 2020;80(18):1901–1928. doi:10.1007/s40265-020-01422-9.
39.
Carlini F, Ivaldi F, Gualandi F, et al. Different susceptibility of T and B cells to cladribine depends on their levels of deoxycytidine kinase activity linked to activation status. J Neuroimmune Pharmacol. 2022;17(1–2):195–205. doi:10.1007/s11481-021-09994-3.
40.
Comi G, Cook S, Giovannoni G, et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:168–174. doi:10.1016/j.msard.2019.01.038.
41.
Nabizadeh F, Mohamadi M, Rahmani S, et al. Safety and efficacy of cladribine in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci. 2023;44(9):3045–3057. doi:10.1007/s10072-023-06794-w.
42.
Bar-Or A, Li R. Cellular immunology of relapsing multiple sclerosis: interactions, checks, and balances. Lancet Neurol. 2021;20(6):470–483. doi:10.1016/S1474-4422(21)00063-6.
43.
Segal BM. The diversity of encephalitogenic CD4+ T cells in multiple sclerosis and its animal models. J Clin Med. 2019;8(1):120. doi:10.3390/jcm8010120.
44.
Jordan AL, Yang J, Fisher CJ, et al. Progressive multifocal leukoencephalopathy in dimethyl fumarate-treated multiple sclerosis patients. Mult Scler. 2022;28(1):7–15. doi:10.1177/1352458520949158.
45.
Genc B, Bozan HR, Genc S, Genc K. Stem cell therapy for multiple sclerosis. Adv Exp Med Biol. 2019;1084:145–174. doi:10.1007/5584_2018_247.
46.
Matsoukas J, Deraos G, Kelaidonis K, et al. Myelin peptide-mannan conjugate multiple sclerosis vaccines: conjugation efficacy and stability of vaccine ingredient. Vaccines (Basel). 2021;9(12):1456. doi:10.3390/vaccines9121456.
47.
Phan NM, Nguyen TL, Min DK, et al. Mesoporous polydopamine nanoparticle-based tolerogenic vaccine induces antigen-specific immune tolerance to prevent and treat autoimmune multiple sclerosis. Biomaterials. 2025;316:122997. doi:10.1016/j.biomaterials.2024.122997.
48.
Bemani P, Jalili S, Hassanpour K, et al. Designing and characterization of Tregitope-based multi-epitope vaccine against multiple sclerosis: an immunoinformatic approach. Curr Drug Saf. 2023;18(1):79–92. doi:10.2174/1574886317666220429105439.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.