Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Pediatric-onset multiple sclerosis (POMS) - diagnosis, treatment and psychological aspects
1
Internship, Franciszek Raszeja City Hospital, Poznań, Poland
2
Internship, Cardinal Stefan Wyszyński Provincial Specialist Hospital, Lublin, Poland
3
Internship, Independent Public Health Care Centre, Łęczna, Poland
4
Scientific Students Association at the Department of Clinical Genetics, Medical University, Lublin, Poland
5
Scientific Students Association at the 1st Department of Medical Radiology, Medical University, Lublin, Poland
6
Internship, Dr. Anna Gostynskaya Wolski Hospital, Warsaw, Poland
J Pre Clin Clin Res. 2025;19(3):117-124
KEYWORDS
POMSmultiple sclerosispaediatric multiple sclerosischildhood multiple sclerosispaediatric onset multiple sclerosis
TOPICS
ABSTRACT
Introduction and objective:
Paediatric-onset multiple sclerosis (POMS) is a chronic, immune-mediated disease of the central nervous system that manifests before the age of 18. Although relatively rare, it poses unique diagnostic and therapeutic challenges due to differences in immune system maturity, disease presentation, and response to treatment compared to adult-onset MS
Review methods:
The review systematically synthesizes evidence from various studies to provide a comprehensive overview of POMS, integrating findings on its etiology, diagnosis, treatment, and psychosocial impact.
Brief description of the state of knowledge:
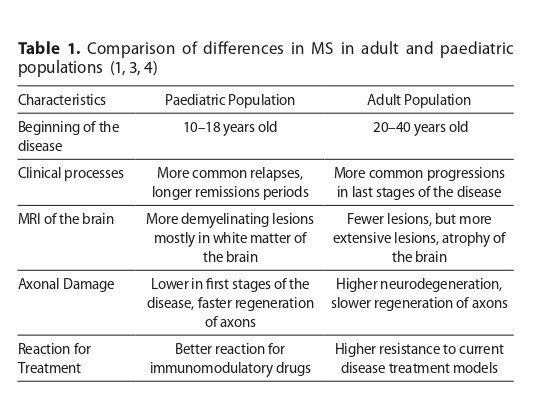
The pathogenesis of POMS involves both T and B cell dysregulation, with particular attention paid to the role of IL-17, Epstein-Barr virus infection, HLA-DRB1 phenotype, and environmental factors such as vitamin D deficiency. Diagnostic accuracy has improved through the use of the 2017 McDonald criteria, MRI imaging, cerebrospinal fluid analysis, and detection of specific antibodies. Treatment approaches range from corticosteroids for acute relapses to long-term disease-modifying therapies. First-line immunomodulatory agents, such as interferon-β and glatiramer acetate, are increasingly supplemented or replaced by modern oral medications (e.g., fingolimod, dimethyl fumarate) and monoclonal antibodies (e.g., rituximab, ocrelizumab, natalizumab). In addition to biomedical management, the disease significantly affects the psychological functioning of patients, their cognitive development, and family dynamics. A holistic, multidisciplinary approach that includes psycho-social support is essential for improving long-term outcomes and quality of life for both the children and their caregivers.
Summary:
The review presents a comprehensive analysis of current knowledge on the etiology, diagnostic methods, treatment strategies, and the psycho-social impact of POMS.
Paediatric-onset multiple sclerosis (POMS) is a chronic, immune-mediated disease of the central nervous system that manifests before the age of 18. Although relatively rare, it poses unique diagnostic and therapeutic challenges due to differences in immune system maturity, disease presentation, and response to treatment compared to adult-onset MS
Review methods:
The review systematically synthesizes evidence from various studies to provide a comprehensive overview of POMS, integrating findings on its etiology, diagnosis, treatment, and psychosocial impact.
Brief description of the state of knowledge:
The pathogenesis of POMS involves both T and B cell dysregulation, with particular attention paid to the role of IL-17, Epstein-Barr virus infection, HLA-DRB1 phenotype, and environmental factors such as vitamin D deficiency. Diagnostic accuracy has improved through the use of the 2017 McDonald criteria, MRI imaging, cerebrospinal fluid analysis, and detection of specific antibodies. Treatment approaches range from corticosteroids for acute relapses to long-term disease-modifying therapies. First-line immunomodulatory agents, such as interferon-β and glatiramer acetate, are increasingly supplemented or replaced by modern oral medications (e.g., fingolimod, dimethyl fumarate) and monoclonal antibodies (e.g., rituximab, ocrelizumab, natalizumab). In addition to biomedical management, the disease significantly affects the psychological functioning of patients, their cognitive development, and family dynamics. A holistic, multidisciplinary approach that includes psycho-social support is essential for improving long-term outcomes and quality of life for both the children and their caregivers.
Summary:
The review presents a comprehensive analysis of current knowledge on the etiology, diagnostic methods, treatment strategies, and the psycho-social impact of POMS.
Zych K, Korga M, Żyła D, Jakubas A, Zuzak AP, Krawczyk M, Koziej S. Paediatric-onset multiple sclerosis (POMS) – diagnosis, treatment and
psychological aspects. J Pre Clin-Clin Res. 2025;19(3):117–124. doi: 10.26444/jpccr/208785
REFERENCES (51)
1.
Yan K, Balijepalli C, Desai K, Gullapalli L, Druyts E. Epidemiology of paediatric multiple sclerosis: A systematic literature review and meta-analysis. Multiple Sclerosis and Related Disorders. 2020;44.
2.
Alroughani R, Boyko A. Paediatric multiple sclerosis: a review. BMC Neurol. 2018;18:27.
3.
Brola W, Steinborn B. Paediatric multiple sclerosis – current status of epidemiology, diagnosis and treatment. Neurol Neurochir Pol. 2020;54(6):508–17.
4.
Comi G, Bar-Or A, Lassmann H, Uccelli A, Hartung HP, Montalban X, et al. The role of B cells in Multiple Sclerosis and related disorders. Ann Neurol. 2021;89(1):13–23.
5.
Palle P, Monaghan KL, Milne SM, Wan ECK. Cytokine Signaling in Multiple Sclerosis and Its Therapeutic Applications. Med Sci (Basel). 2017;5(4):23.
6.
Ruprecht K. The role of Epstein-Barr virus in the etiology of multiple sclerosis: a current review. Expert Rev Clin Immunol. 2020;16(12):1143–57.
7.
Mey GM, Mahajan KR, DeSilva TM. Neurodegeneration in multiple sclerosis. WIREs Mech Dis. 2023;15(1):e1583.
8.
Balasooriya NN, Elliott TM, Neale RE, Vasquez P, Comans T, Gordon LG. The association between vitamin D deficiency and multiple sclerosis: an updated systematic review and meta-analysis. Multiple Sclerosis and Related Disorders. 2024;90:105804.
9.
Li EC, Zheng Y, Cai MT, Lai QL, Fang GL, Du BQ, et al. Seizures and epilepsy in multiple sclerosis, aquaporin 4 antibody-positive neuromyelitis optica spectrum disorder, and myelin oligodendrocyte glycoprotein antibody-associated disease. Epilepsia. 2022;63(9):2173–91.
10.
Hacohen Y, Brownlee W, Mankad K, Chong WK ‘Kling’, Thompson A, Lim M, et al. Improved performance of the 2017 McDonald criteria for diagnosis of multiple sclerosis in children in a real-life cohort. Mult Scler. 2020;26(11):1372–80.
11.
Fadda G, Brown RA, Longoni G, Castro DA, O’Mahony J, Verhey LH, et al. MRI and laboratory features and the performance of international criteria in the diagnosis of multiple sclerosis in children and adolescents: a prospective cohort study. Lancet Child Adolesc Health. 2018;2(3):191–204.
12.
Shahriari M, Sotirchos ES, Newsome SD, Yousem DM. MOGAD: How It Differs From and Resembles Other Neuroinflammatory Disorders. AJR Am J Roentgenol. 2021;216(4):1031–9.
13.
Wei S, Xu L, Zhou D, Wang T, Liu K, Gao F, et al. Differentiation of MOGAD in ADEM-like presentation children based on FLAIR MRI features. Mult Scler Relat Disord. 2023;70:104496.
14.
Wong YYM, de Mol CL, van der Vuurst de Vries RM, van Pelt ED, Ketelslegers IA, Catsman-Berrevoets CE, et al. Real-world validation of the 2017 McDonald criteria for paediatric MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(2):e528.
15.
Wattjes MP, Ciccarelli O, Reich DS, Banwell B, de Stefano N, Enzinger C, et al. 2021 MAGNIMS-CMSC-NAIMS consensus recommendations on the use of MRI in patients with multiple sclerosis. Lancet Neurol. 2021;20(8):653–70.
16.
Machado-Rivas F, Jaimes C, Scherrer B, Benson LA, Gorman MP, Warfield SK, et al. Evaluation of white matter microstructure in paediatric onset multiple sclerosis with diffusion compartment imaging. J Neuroimaging. 2022;32(6):1098–108.
17.
de Mol CL, Neuteboom RF, Jansen PR, White T. White matter microstructural differences in children and genetic risk for multiple sclerosis: A population-based study. Mult Scler. 2022;28(5):730–41.
18.
McKay KA, Wickström R, Hillert J, Karrenbauer VD. Cerebrospinal fluid markers in incident paediatric-onset multiple sclerosis: a nationwide study. Sci Rep. 2021;11(1):18528.
19.
Martinez B, Peplow PV. MicroRNAs in blood and cerebrospinal fluid as diagnostic biomarkers of multiple sclerosis and to monitor disease progression. Neural Regen Res. 2020;15(4):606–19.
20.
Bianchi A, Cortese R, Prados F, Tur C, Kanber B, Yiannakas MC, et al. Optic chiasm involvement in multiple sclerosis, aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder and myelin oligodendrocyte glycoprotein-associated disease. Mult Scler. 2024;30(6):674–86.
21.
Banwell B, Bennett JL, Marignier R, Kim HJ, Brilot F, Flanagan EP, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023;22(3):268–82.
22.
Marignier R, Hacohen Y, Cobo-Calvo A, Pröbstel AK, Aktas O, Alexopoulos H, et al. Myelin-oligodendrocyte glycoprotein antibody-associated disease. Lancet Neurol. 2021;20(9):762–72.
23.
Lattanzi S, Cagnetti C, Danni M, Provinciali L, Silvestrini M. Oral and intravenous steroids for multiple sclerosis relapse: a systematic review and meta-analysis. J Neurol. 2017;264(8):1697–704.
24.
Mavridi A, Bompou ME, Redmond A, Archontakis-Barakakis P, Vavougios GD, Mitsikostas DD, et al. Current and Emerging Treatment Options in Paediatric Onset Multiple Sclerosis. Sclerosis. 2024;2(2):88–107.
25.
Xavier A, Campagna MP, Maltby VE, Kilpatrick T, Taylor BV, Butzkueven H, et al. Interferon beta treatment is a potent and targeted epigenetic modifier in multiple sclerosis. Front Immunol. 2023;14:1162796.
26.
Skarlis C, Markoglou N, Gontika M, Bougea A, Katsavos S, Artemiadis A, et al. First-line disease modifying treatments in paediatric-onset multiple sclerosis in Greece: therapy initiation at more advanced age is the main cause of treatment failure, in a retrospective observational study, with a cohort from a single Multiple Sclerosis Center. Neurol Sci. 2023;44(2):693–701.
27.
Kasindi A, Fuchs DT, Koronyo Y, Rentsendorj A, Black KL, Koronyo-Hamaoui M. Glatiramer Acetate Immunomodulation: Evidence of Neuroprotection and Cognitive Preservation. Cells. 2022;11(9):1578.
28.
Teva Takeda Pharma Ltd. Copaxone Subcutaneous Injection Syringe Special Drug Use-Result Investigation (All-Case Investigation) ‘Prevention of Relapse of Multiple Sclerosis’. clinicaltrials.gov. 2024. Report No.: NCT03209479.
29.
IMPULS Endowment Fund. Czech Pharmaco-epidemiological Real World Data Study Focused on Effectiveness of Different Disease Modifying Drugs. clinicaltrials.gov. 2023. Report No.: NCT05762003.
30.
Yang T, Tian X, Chen CY, Ma LY, Zhou S, Li M, et al. The efficacy and safety of fingolimod in patients with relapsing multiple sclerosis: A meta-analysis. Br J Clin Pharmacol. 2020;86(4):637–45.
31.
Krupp L, Banwell B, Chitnis T, Deiva K, Gaertner J, Ghezzi A, et al. Effect of fingolimod on health-related quality of life in paediatric patients with multiple sclerosis: results from the phase 3 PARADIGMS Study. BMJ Neurol Open. 2022;4(1):e000215.
32.
Arnold DL, Banwell B, Bar-Or A, Ghezzi A, Greenberg BM, Waubant E, et al. Effect of fingolimod on MRI outcomes in patients with paediatric-onset multiple sclerosis: results from the phase 3 PARADIGMS study. J Neurol Neurosurg Psychiatry. 2020;91(5):483–92.
33.
Højsgaard Chow H, Talbot J, Lundell H, Marstrand L, Gøbel Madsen C, Bach Søndergaard H, et al. Dimethyl fumarate treatment of primary progressive multiple sclerosis: results of an open-label extension study. Mult Scler Relat Disord. 2023;70:104458.
34.
Vermersch P, Scaramozza M, Levin S, Alroughani R, Deiva K, Pozzilli C, et al. Effect of Dimethyl Fumarate vs Interferon β-1a in Patients With Paediatric-Onset Multiple Sclerosis: The CONNECT Randomized Clinical Trial. JAMA Netw Open. 2022;5(9):e2230439.
35.
Filippini G, Kruja J, Del Giovane C. Rituximab for people with multiple sclerosis. Cochrane Database Syst Rev. 2021;11(11):CD013874.
36.
Breu M, Sandesjö F, Milos RI, Svoboda J, Salzer J, Schneider L, et al. Rituximab treatment in paediatric-onset multiple sclerosis. European Journal of Neurology. 2024;31(5):e16228.
37.
Riera R, Torloni MR, Martimbianco ALC, Pacheco RL. Alemtuzumab for multiple sclerosis. Cochrane Database Syst Rev. 2023;6(6):CD011203.
38.
Bibinoğlu Amirov C, Saltık S, Yalçınkaya C, Tütüncü M, Saip S, Siva A, et al. Ocrelizumab in paediatric multiple sclerosis. European Journal of Paediatric Neurology. 2023;43:1–5.
39.
Mar S, Valeriani M, Steinborn B, Schreiner T, Waubant E, Filippi M, et al. Ocrelizumab dose selection for treatment of paediatric relapsing–remitting multiple sclerosis: results of the OPERETTA I study. J Neurol. 2025;272(2):137.
40.
Samjoo IA, Drudge C, Walsh S, Tiwari S, Brennan R, Boer I, et al. Comparative efficacy of therapies for relapsing multiple sclerosis: a systematic review and network meta-analysis. J Comp Eff Res. 2023;12(7):e230016.
41.
Palavra F, Silva D, Fernandes C, Faustino R, Vasconcelos M, Pereira C, et al. Clinical predictors of NEDA-3 one year after diagnosis of paediatric multiple sclerosis: an exploratory single-center study. Front Neurosci. 2023;17.
42.
Hauser SL, Cross AH, Winthrop K, Wiendl H, Nicholas J, Meuth SG, et al. Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult Scler. 2022;28(10):1576–90.
43.
Tarantino S, Proietti Checchi M, Papetti L, Monte G, Ferilli MAN, Valeriani M. Neuropsychological performances, quality of life, and psychological issues in paediatric onset multiple sclerosis: a narrative review. Neurol Sci. 2024;45(5):1913–30.
44.
Ghai S, Kasilingam E, Lanzillo R, Malenica M, van Pesch V, Burke NC, et al. Needs and Experiences of Children and Adolescents with Paediatric Multiple Sclerosis and Their Caregivers: A Systematic Review. Children (Basel). 2021;8(6):445.
45.
Mrosková S, Klímová E, Majerníková Ľ, Tkáčová Ľ. Quality of Life of Children and Adolescents with Multiple Sclerosis – A Literature Review of the Quantitative Evidence. Int J Environ Res Public Health. 2021;18(16):8645.
46.
Aloni R, Asher G, Ben-Ari A, Menascu S. Unveiling the Psychological Consequences of Illness Perception in Paediatric Multiple Sclerosis: A Parent-Child Study. Children (Basel). 2024;11(8):929.
47.
Tarantino S, Proietti Checchi M, Papetti L, Monte G, Ferilli MAN, Valeriani M. Parental Experiences in Paediatric Multiple Sclerosis: Insights from Quantitative Research. Children (Basel). 2024;11(1):71.
48.
Kaplan SH, Shaughnessy M, Fortier MA, Vivero-Montemayor M, Masague SG, Hayes D, et al. The role of parental health and distress in assessing children’s health status. Qual Life Res. 2022;31(12):3403–12.
49.
O’Mahony J, Marrie RA, Laporte A, Brown A. Addressing Health-Related Quality of Life Among Children With Multiple Sclerosis. Int J MS Care. 2023;25(1):35–42.
50.
Ehtesham N, Rafie MZ, Mosallaei M. The global prevalence of familial multiple sclerosis: an updated systematic review and meta-analysis. BMC Neurol. 2021;21:246.
51.
Cahyadi M, Mesinovic J, Chim ST, Ebeling P, Zengin A, Grech L. Medication and bone health in multiple sclerosis: A systematic review and meta-analysis. J Manag Care Spec Pharm. 2023;29(12):1331–53.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.