RESEARCH PAPER
Three-drug combination of lacosamide, phenobarbital and valproate exerts additive interaction in the tonic-clonic seizure model in mice
1
Department of Pathophysiology, Medical University, Lublin, Poland
2
Department of Toxicology and Food Safety, Institute of Rural Health, Lublin, Poland
3
Department and Clinic of Hematolooncology and Bone Marrow Transplantation, Medical University, Lublin, Poland
4
Department and Clinic of Cardiology, Medical University, Lublin, Poland
5
Department of Orthopedics and Traumatology, Medical University, Lublin, Poland
6
Isobolographic Analysis Laboratory, Institute of Rural Health, Lublin, Poland
Corresponding author
Jarogniew J. Łuszczki
Department of Pathophysiology, Medical University of Lublin, 20-090, Lublin, Poland
Department of Pathophysiology, Medical University of Lublin, 20-090, Lublin, Poland
J Pre Clin Clin Res. 2020;14(3):102-106
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Triple-therapy with antiepileptic drugs (AEDs) is usually prescribed for epilepsy patients, whose seizures are not fully controlled with standard medications. Although 25 various AEDs are currently licensed for treating epilepsy, no algorithms allowing for the proper combination of AEDs are available.
Objective:
The aim of the study is to isobolographically assess the type of interaction among three AEDs (lacosamide [LCM], phenobarbital [PB] and valproate [VPA]), in the model of tonic-clonic seizures in mice.
Material and methods:
The electrically-evoked (25 mA, 500 V, 50 Hz, 0.2 s of stimulus duration) tonic-clonic seizures in male albino Swiss mice allowed determination of the anticonvulsant action of the three-drug mixture of LCM, PB and VPA combined in a dose ratio of 1:1:1 by means of type I isobolographic analysis of interaction.
Results:
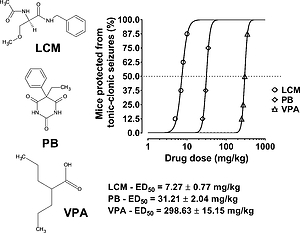
The experimentally-determined ED50 exp value for the three-drug mixture was 112.04 mg/kg and did not differ from the theoretically calculated ED50 add value, which was 112.36 mg/kg. Lack of statistical significance confirmed that the mixture of LCM, PB and VPA in a dose-ratio of 1:1:1 exerted additive interaction in the mouse tonic-clonic seizure model.
Conclusions:
Although the three-drug combination of LCM, PB and VPA produced additive interaction in the mouse tonic-clonic seizure model, the three-drug combination could be recommended for epilepsy patients whose seizures are refractory to the standard medication.
Triple-therapy with antiepileptic drugs (AEDs) is usually prescribed for epilepsy patients, whose seizures are not fully controlled with standard medications. Although 25 various AEDs are currently licensed for treating epilepsy, no algorithms allowing for the proper combination of AEDs are available.
Objective:
The aim of the study is to isobolographically assess the type of interaction among three AEDs (lacosamide [LCM], phenobarbital [PB] and valproate [VPA]), in the model of tonic-clonic seizures in mice.
Material and methods:
The electrically-evoked (25 mA, 500 V, 50 Hz, 0.2 s of stimulus duration) tonic-clonic seizures in male albino Swiss mice allowed determination of the anticonvulsant action of the three-drug mixture of LCM, PB and VPA combined in a dose ratio of 1:1:1 by means of type I isobolographic analysis of interaction.
Results:
The experimentally-determined ED50 exp value for the three-drug mixture was 112.04 mg/kg and did not differ from the theoretically calculated ED50 add value, which was 112.36 mg/kg. Lack of statistical significance confirmed that the mixture of LCM, PB and VPA in a dose-ratio of 1:1:1 exerted additive interaction in the mouse tonic-clonic seizure model.
Conclusions:
Although the three-drug combination of LCM, PB and VPA produced additive interaction in the mouse tonic-clonic seizure model, the three-drug combination could be recommended for epilepsy patients whose seizures are refractory to the standard medication.
Kondrat-Wróbel M, Marzęda P, Bojar H, Wróblewska-Łuczka P, Kozińska J, Jankiewicz M, Kominek M, J. Łuszczki J.Three-drug combination of lacosamide, phenobarbital and valproate exerts additive interaction in the tonic-clonic seizure model in mice. J Pre Clin Clin Res. 2020; 14(3): 102–106. doi: 10. 264 4 4/jpccr/127377
REFERENCES (37)
1.
Sperling MR, Klein P, Aboumatar S, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia 2020; 61: 1099–108. doi: 10.1111/epi.16525.
2.
Yamamoto T, Lim SC, Ninomiya H, et al. Efficacy and safety of perampanel monotherapy in patients with focal-onset seizures with newly diagnosed epilepsy or recurrence of epilepsy after a period of remission: The open-label Study 342 (FREEDOM Study). Epilepsia Open 2020; 5: 274–84. doi:10.1002/epi4.12398.
3.
Kobayashi K, Endoh F, Ohmori I, et al. Action of antiepileptic drugs on neurons. Brain Dev 2020; 42: 2–5. doi:10.1016/j.braindev.2019.07.006
4.
Perucca E, Brodie MJ, Kwan P, et al. 30 years of second-generation antiseizure medications: impact and future perspectives. Lancet Neurol 2020; 19: 544–56. doi: 10.1016/S1474-4422(20)30035-1.
5.
Stephen LJ, Brodie MJ. Antiepileptic drug monotherapy versus polytherapy: pursuing seizure freedom and tolerability in adults. Curr Opin Neurol 2012; 25: 164–72. doi: 10.1097/WCO.0b013e328350ba68.
6.
Luszczki JJ, Czuczwar SJ. Biphasic characteristic of interactions between stiripentol and carbamazepine in the mouse maximal electroshock-induced seizure model: a three-dimensional isobolographic analysis. Naunyn Schmiedebergs Arch Pharmacol 2006; 374: 51–64. doi:10.1007/s00210-006-0100-3.
7.
Luszczki JJ, Trojnar MK, Ratnaraj N, et al. Interactions of stiripentol with clobazam and valproate in the mouse maximal electroshock-induced seizure model. Epilepsy Res 2010; 90: 188–98. doi: 10.1016/j.eplepsyres.2010.04.006.
8.
Tallarida RJ. Quantitative methods for assessing drug synergism. Genes Cancer 2011; 2: 1003–8. doi: 10.1177/1947601912440575.
9.
Łuszczki JJ, Kondrat-Wróbel M, Zagaja M, et al. Sub-additive (antagonistic) interaction of lacosamide with lamotrigine and valproate in the maximal electroshock-induced seizure model in mice: an isobolographic analysis. Pharmacol Rep 2020. doi: 10.1007/s43440-020 - 0 0117-y.
10.
Kondrat-Wróbel MW, Załuska K, Walczak A, et al. Antagonistic interaction of lacosamide with carbamazepine and valproate in the mouse tonic-clonic seizure model. Heal Probl Civiliz 2019; 13: 92–8. doi: 10.5114/hpc.2019.81105.
11.
Załuska K, Kondrat-Wróbel MW, Panasiuk-Poterek AN, et al. Synergy among oxcarbazepine, pregabalin and topiramate in the mouse maximal electroshock-induced seizure test – an isobolographic analysis. J Pre-Clin Clin Res 2018; 12: 111–6. doi: 10.26444/jpccr/101578.
12.
Luszczki JJ, Zagaja M, Miziak B, et al. Beneficial combination of lacosamide with retigabine in experimental animals: an isobolographic analysis. Pharmacology 2018; 101: 22–8. doi: 10.1159/000480019.
13.
Maria Kondrat-Wróbel, Paweł Marzęda, Hubert Bojar, Paula Wróblewska-Łuczka, Justyna Kozińska, Marek Jankiewicz et al. Three-drug combination of lacosamide...13. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Exp Physiol 2020. doi: 10.1113/EP088870.
14.
Stepien KM, Tomaszewski M, Luszczki JJ, et al. The interactions of atorvastatin and fluvastatin with carbamazepine, phenytoin and valproate in the mouse maximal electroshock seizure model. Eur J Pharmacol 2011; 674: 20– 6. doi: 10.1016/j.ejphar.2011.10.030.
15.
Nieoczym D, Łuszczki JJ, Czuczwar SJ, et al. Effect of sildenafil on the anticonvulsant action of classical and second-generation antiepileptic drugs in maximal electroshock-induced seizures in mice. Epilepsia 2010; 51: 1552–9. doi: 10.1111/j.1528-1167.2009.02485.x.
16.
Litchfield JT, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949; 96: 99–113.
17.
Luszczki JJ, Filip D, Czuczwar SJ. Additive interactions of pregabalin with lamotrigine, oxcarbazepine and topiramate in the mouse maximal electroshock-induced seizure model: A type I isobolographic analysis for non-parallel dose-response relationship curves. Epilepsy Res. 2010; 91: 166 –75. doi: 10.1016/j.eplepsyres.2010.07.009.
18.
Luszczki JJ, Andres MM, Czuczwar P, et al. Pharmacodynamic and pharmacokinetic characterization of interactions between levetiracetam and numerous antiepileptic drugs in the mouse maximal electroshock seizure model: An isobolographic analysis. Epilepsia. 2006; 47: 10–20. doi: 10.1111/j.1528-1167.20 06.0 036 4.x.
19.
Tallarida RJ. Drug combinations: tests and analysis with isoboles. Curr Protoc Pharmacol 2016; 72: 9.19.1–9.19.19. doi: 10.1002/0471141755.ph0919s72.
20.
Lederer S, Dijkstra TMH, Heskes T. Additive dose response models: explicit formulation and the Loewe additivity consistency condition. Front Pharmacol 2018; 9: 31. doi: 10.3389/fphar.2018.00031.
21.
Hitiris N, Mohanraj R, Norrie J, et al. Predictors of pharmacoresistant epilepsy. Epilepsy Res 2007; 75: 192–6. doi: 10.1016/j.eplepsyres.2007.06.003.
22.
Brodie MJ, Sills GJ. Combining antiepileptic drugs--rational polytherapy? Seizure 2011; 20: 369–75. doi: 10.1016/j.seizure.2011.01.004.
23.
Perucca E. Pharmacological principles as a basis for polytherapy. Acta Neurol Scand Suppl. 1995; 162: 31–4. doi: 10.1111/j.1600-0404.1995.tb00497.x.
24.
Canevini MP, De Sarro G, Galimberti CA, et al. Relationship between adverse effects of antiepileptic drugs, number of coprescribed drugs, and drug load in a large cohort of consecutive patients with drug-refractory epilepsy. Epilepsia. 2010; 51: 797–804. doi: 10.1111/j.1528-1167.2010.02520.x.
25.
Palleria C, Di Paolo A, Giofrè C, et al. Pharmacokinetic drug-drug interaction and their implication in clinical management. J Res Med Sci 2013; 18: 601–10.
26.
van Dijkman SC, Rauwé WM, Danhof M, et al. Pharmacokinetic interactions and dosing rationale for antiepileptic drugs in adults and children. Br J Clin Pharmacol 2018; 84: 97–111. doi: 10.1111/bcp.13400.
27.
Łuszczki JJ. Third-generation antiepileptic drugs: mechanisms of action, pharmacokinetics and interactions. Pharmacol Rep 2009; 61: 197–216.doi: 10.1016/s1734-1140(09)70024-6.
28.
Brodie MJ, Kwan P. Current position of phenobarbital in epilepsy and its future. Epilepsia. 2012; 53 Suppl 8: 40–6. doi:10.1111/epi.12027.
29.
Tomson T, Battino D, Perucca E. Valproic acid after five decades of use in epilepsy: time to reconsider the indications of a time-honoured drug. Lancet Neurol. 2016; 15: 210–8. doi: 10.1016/S1474-4422(15)00314-2.
30.
Kondrat-Wróbel MW, Łuszczki JJ. Interaction of three-drug combination of lacosamide, carbamazepine and phenobarbital in the mouse maximal electroshock-induced seizure model – an isobolographic analysis. Heal Probl Civiliz. 2016; 10: 55–61. doi: 10.5114/hpc.2016.58209.
31.
Kondrat-Wróbel MW, Łuszczki JJ. Additive interaction for three-drug combination of carbamazepine, lacosamide and lamotrigine against maximal electroshock-induced seizures – a type I isobolographic analysis. Eur J Clin Exp Med. 2017; 15: 303–9. doi: 10.15584/ejcem.2017.4.1.
32.
Kondrat-Wróbel MW, Łuszczki JJ. Isobolographic additivity among lacosamide, lamotrigine and phenobarbital in a mouse tonic-clonic seizure model. Adv Clin Exp Med. 2018; 27: 881–6. doi: 10.17219/acem/69132.
33.
Luszczki JJ. Isobolographic analysis of interaction for three-drug combination of carbamazepine, phenobarbital and topiramate in the mouse maximal electroshock-induced seizure model. Pharmacology 2016; 97: 259–64. doi: 10.1159/000444452.
34.
Luszczki JJ, Mazurkiewicz LP, Wroblewska-Luczka P, et al. Combination of phenobarbital with phenytoin and pregabalin produces synergy in the mouse tonic-clonic seizure model: An isobolographic analysis. Epilepsy Res. 2018; 145: 116–22. doi: 10.1016/j.eplepsyres.2018.06.003.
35.
Załuska K, Marzęda P, Bojar H, et al. Additive suppression of tonic-clonic seizures in mice receiving the combination of carbamazepine, phenobarbital and valproate. J Pre-Clin Clin Res. 2019; 13: 72–5. doi: 10.26444/jpccr/109381.
36.
Stephen LJ, Brodie MJ. Seizure freedom with more than one antiepileptic drug. Seizure 2002; 11: 349–51. doi: 10.1053/seiz.2002.0711.
37.
Stephen LJ, Forsyth M, Kelly K, et al. Antiepileptic drug combinations--have newer agents altered clinical outcomes? Epilepsy Res. 2012; 98: 194–8. doi: 10.1016/j.eplepsyres.2011.09.008.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.