Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Additivity interactions between fluconazole and citrus essential oils to Aspergillus fumigatus
1
Department of Pathophysiology, Medical University, Lublin, Poland
Corresponding author
Paula Wróblewska-Łuczka
Department of Pathophysiology, Medical University of Lublin, Jaczewskiego, 8b, 20-090, Lublin, Poland
Department of Pathophysiology, Medical University of Lublin, Jaczewskiego, 8b, 20-090, Lublin, Poland
J Pre Clin Clin Res. 2021;15(3):116-120
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Aspergillus fumigatus is the most common pathogen causing allergic bronchopulmonary mycosis. The pathogenic capacity of Aspergillus fumigatus is related to its thermal tolerance and the small size of the spores which enables transfer to the respiratory tract. In the case of fungal diseases, their treatment is based on fungicidal antibiotics, such as fluconazole. Due to the growing problem of drug resistance, new therapeutic solutions are sought, especially of natural origin. Essential oils, due to their anti-bacterial, anti-fungal, anti-inflammatory and immunostimulatory properties, constitute interesting research material in the fight against mould.
Objective:
The aim of the study was to assess the type of pharmacodynamic interactions between fluconazole and selected essential oils: lemon, orange, tangerine, and grapefruit in an in vitro study against Aspergillus fumigatus. Isobolographic analysis of the results allowed determining the type of interactions between fluconazole and the tested essential oils.
Results:
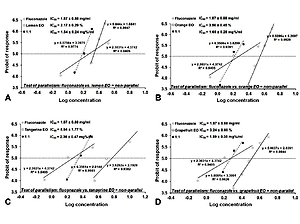
According to the research results, a IC50 dose of fluconazole versus Aspergillus fumigatus IC50=1.87±0.88 mg/ml. The most active essential oil was lemon oil, which at the concentration of 4% in medium completely inhibited the growth of Aspergillus fumigatus. Tangerine essential oil is the least active against A. fumigatus. Isobolographic analysis of the interactions between fluconazole and essential oils showed additive interactions for the combination of fluconazole with lemon, orange and grapefruit ols, and an additive interaction with a tendency to synergism for the combination of fluconazole with tangerine oil.
Conclusions:
Isobographic analysis can contribute to the introduction of natural substances into the therapy of many diseases.
Aspergillus fumigatus is the most common pathogen causing allergic bronchopulmonary mycosis. The pathogenic capacity of Aspergillus fumigatus is related to its thermal tolerance and the small size of the spores which enables transfer to the respiratory tract. In the case of fungal diseases, their treatment is based on fungicidal antibiotics, such as fluconazole. Due to the growing problem of drug resistance, new therapeutic solutions are sought, especially of natural origin. Essential oils, due to their anti-bacterial, anti-fungal, anti-inflammatory and immunostimulatory properties, constitute interesting research material in the fight against mould.
Objective:
The aim of the study was to assess the type of pharmacodynamic interactions between fluconazole and selected essential oils: lemon, orange, tangerine, and grapefruit in an in vitro study against Aspergillus fumigatus. Isobolographic analysis of the results allowed determining the type of interactions between fluconazole and the tested essential oils.
Results:
According to the research results, a IC50 dose of fluconazole versus Aspergillus fumigatus IC50=1.87±0.88 mg/ml. The most active essential oil was lemon oil, which at the concentration of 4% in medium completely inhibited the growth of Aspergillus fumigatus. Tangerine essential oil is the least active against A. fumigatus. Isobolographic analysis of the interactions between fluconazole and essential oils showed additive interactions for the combination of fluconazole with lemon, orange and grapefruit ols, and an additive interaction with a tendency to synergism for the combination of fluconazole with tangerine oil.
Conclusions:
Isobographic analysis can contribute to the introduction of natural substances into the therapy of many diseases.
ABBREVIATIONS
EO – essential oil; IC50 – median inhibitory concentration; IC50 mix – median inhibitory concentration for the mixture of the tested substances; PDA – Potato Dextrose Agar medium
Wróblewska-Łuczka P, Łuszczki J. Additivity interactions between fluconazole and citrus essential oils to Aspergillus fumigatus. J Pre-Clin Clin
Res. 2021; 15(3): 116–120. doi: 10.26444/jpccr/140077
REFERENCES (49)
1.
Agbetile J, Fairs A, Desai D, et al. Isolation of filamentous fungi from sputum in asthma is associated with reduced post-bronchodilator FEV1. Clin Exp Allergy. 2012; 42: 782–791. doi: 10.1111/j.1365-2222.2012.03987.x.
2.
Hérivaux A, Gonçalves SM, Carvalho A, Cunha C. Microbiota-derived metabolites as diagnostic markers for respiratory fungal infections. J Pharm Biomed Anal. 2020; 189: 113473. doi: https://doi.org/10.1016/j.jpba....
3.
Baxter CG, Moore CB, Jones AM, et al. IgE-mediated immune responses and airway detection of Aspergillus and Candida in adult cystic fibrosis. Chest. 2013; 143: 1351–1357. doi: 10.1378/chest.12-1363.
4.
Keown K, Reid A, Moore JE, Taggart CC, Downey DG. Coinfection with Pseudomonas aeruginosa and Aspergillus fumigatus in cystic fibrosis. Eur Respir Rev. 2020; 29(158): 200011. doi: 10.1183/16000617.0011-2020.
5.
Bafadhel M, McKenna S, Agbetile J, et al. Aspergillus fumigatus during stable state and exacerbations of COPD. Eur Respir J. 2014; 43: 64–71. doi: 10.1183/09031936.00162912.
6.
Dhooria S, Kumar P, Saikia B, et al. Prevalence of Aspergillus sensitisation in pulmonary tuberculosis-related fibrocavitary disease. Int J Tuberc Lung Dis. 2014; 18: 850–855. doi: 10.5588/ijtld.13.0838.
7.
Wassano NS, Goldman GH, Damasio A. Aspergillus fumigatus. Trends Microbiol. 2020; 28(7): 594–595. doi: 10.1016/j.tim.2020.02.013.
8.
Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus – what makes the species a ubiquitous human fungal pathogen? PLoS Pathog 2013; 9: e1003743. doi: 10.1371/journal.ppat.1003743.
9.
van de Veerdonk FL, Gresnigt MS, Romani L, et al. Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol. 2017; 15(11): 661–674. doi: 10.1038/nrmicro.2017.90.
10.
Russo A, Tiseo G, Falcone M, Menichetti F. Pulmonary Aspergillosis: An Evolving Challenge for Diagnosis and Treatment. Infect Dis Ther. 2020; 9(3): 511–524. doi: 10.1007/s40121-020–00315-4.
11.
Latgé JP, Chamilos G. Aspergillus fumigatus and Aspergillosis in 2019. Clin Microbiol Rev. 2019; 33(1): e00140–18. doi: 10.1128/CMR.00140-18.
12.
Wiederhold NP. Antifungal resistance: current trends and future strategies to combat. Infect Drug Resist. 2017; 10: 249–259. doi: 10.2147/IDR .S124918.
13.
Guinea J. Updated EUCAST Clinical Breakpoints against Aspergillus, Implications for the Clinical Microbiology Laboratory. J Fungi (Basel). 2020; 6(4): 343. doi: 10.3390/jof6040343.
14.
Hocayen PAS, Wendler E, Vecchia DD, et al. The nitrergic neurotransmission contributes to the anxiolytic-like effect of Citrus sinensis essential oil in animal models. Phytother Res. 2019; 33(4): 901–909. doi: 10.1002/ptr.6281.
15.
Falzon CC, Balabanova A. Phytotherapy: An Introduction to Herbal Medicine. Prim Care. 2017; 44(2): 217–227. doi: 10.1016/j.pop.2017.02.001.
16.
Colalto C. What phytotherapy needs: Evidence-based guidelines for better clinical practice. Phytother Res. 2018; 32(3): 413–425. doi: 10.1002/ptr.5977.
17.
Geraci A, Di Stefano V, Di Martino E, et al. Essential oil components of orange peels and antimicrobial activity. Nat Prod Res. 2017; 31(6): 653– 659. doi: 10.1080/14786419.2016.1219860.
18.
Ambrosio CMS, Ikeda NY, Miano AC, et al. Unraveling the selective antibacterial activity and chemical composition of citrus essential oils. Sci Rep. 2019; 9(1): 17719. doi: 10.1038/s41598-019-54084-3.
19.
Suroowan S, Mahomoodally MF. Herbal Medicine of the 21st Century: A Focus on the Chemistry, Pharmacokinetics and Toxicity of Five Widely Advocated Phytotherapies. Curr Top Med Chem. 2019; 19(29): 2718–2738. doi: 10.2174/1568026619666191112121330.
20.
Clements ND, Connolly BR, Dicks MA, Mullur RS. The Use of Vitamins, Supplements, Herbs, and Essential Oils in Rehabilitation. Phys Med Rehabil Clin N Am. 2020; 31(4): 685–697. doi: 10.1016/j.pmr.2020.07.010.
21.
Diniz do Nascimento L, Moraes AAB, Costa KSD, et al. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules. 2020; 10(7): 988. doi: 10.3390/biom10070988.
22.
Yap PS, Yiap BC, Ping HC, Lim SH. Essential oils, a new horizon in combating bacterial antibiotic resistance. Open Microbiol J. 2014; 8: 6 –14. doi: 10.2174/1874285801408010006.
23.
Quirino A, Morelli P, Capua G, et al. Synergistic and antagonistic effects of Citrus bergamia distilled extract and its major components on drug resistant clinical isolates. Nat Prod Res. 2020 Jun; 34(11): 1626–1629. doi: 10.1080/14786419.2018.1522631.
24.
Wińska K, Mączka W, Łyczko J, et al. Essential Oils as Antimicrobial Agents-Myth or Real Alternative? Molecules. 2019; 24(11): 2130. doi: 10.3390/molecules24112130.
25.
Yap PS, Lim SH, Hu CP, Yiap BC. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine. 2013; 20(8–9): 710–3. doi: 10.1016/j.phymed.2013.02.013.
26.
Wróblewska-Łuczka P. Isobolographic in vitro interactions of fluconazole with citrus essential oils against Cladosporium cladosporioides. J Pre Clin Clin Res. 2021. doi: https://doi.org/10.26444/jpccr....
27.
Elefanti A, Mouton JW, Verweij PE, et al. Amphotericin B- and voriconazole-echinocandin combinations against Aspergillus spp.: Effect of serum on inhibitory and fungicidal interactions. Antimicrob Agents Chemother. 2013; 57(10): 4656–4663. doi: 10.1128/AAC.00597-13.
28.
Meletiadis J, te Dorsthorst DT, Verweij PE. The concentration-dependent nature of in vitro amphotericin B-itraconazole interaction against Aspergillus fumigatus: isobolographic and response surface analysis of complex pharmacodynamic interactions. Int J Antimicrob Agents. 2006; 28(5): 439–449. doi: 10.1016/j.ijantimicag.2006.07.011.
29.
Stergiopoulou T, Meletiadis J, Sein T, et al. Isobolographic analysis of pharmacodynamic interactions between antifungal agents and ciprofloxacin against Candida albicans and Aspergillus fumigatus. Antimicrob Agents Chemother. 2008; 52(6): 2196–204. doi: 10.1128/AAC.00735-07.
30.
Stergiopoulou T, Meletiadis J, Sein T, et al. Comparative pharmacodynamic interaction analysis between ciprofloxacin, moxifloxacin and levofloxacin and antifungal agents against Candida albicans and Aspergillus fumigatus. J Antimicrob Chemother. 2009; 63(2): 343–348. doi: 10.1093/jac/dkn473.
31.
Ferro BE, Meletiadis J, Wattenberg M, et al. Clofazimine prevents the regrowth of Mycobacterium abscessus and Mycobacterium avium type strains exposed to amikacin and clarithromycin. Antimicrob Agents Chemother. 2015; 60(2): 1097–1105. doi: https://doi.org/10.1128/AAC.02....
32.
Aloui H, Khwaldia K, Licciardello F, et al. Efficacy of the combined application of chitosan and Locust Bean Gum with different citrus essential oils to control postharvest spoilage caused by Aspergillus flavus in dates. Int J Food Microbiol. 2014; 170: 21–28. doi: https://doi.org/10.1016/j.ijfo....
33.
Budzyńska A, Więckowska-Szakiel M, Kalemba D, et al. The optimization of methods utilized for testing the antibacterial activity of essential oils. Med Dośw Mikrobiol. 2009; 61: 281–287.
34.
Litchfield JT Jr, Wilcoxon F. A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther. 1949; 96: 99–113.
35.
Łuszczki JJ. Isobolographic analysis of interaction between drugs with nonparallel dose-response relationship curves: a practical application. Naunyn Schmiedebergs Arch Pharmacol. 2007; 375: 105–114. https://doi.org/10.1007/s00210....
36.
Luszczki JJ, Mazurkiewicz LP, Wroblewska-Luczka P, et al. Combination of phenobarbital with phenytoin and pregabalin produces synergy in the mouse tonic-clonic seizure model: An isobolographic analysis. Epilepsy Res. 2018; 145: 116–122. doi: 10.1016/j.eplepsyres.2018.06.003.
37.
Luszczki JJ, Zagaja M, Miziak B, et al. Beneficial Combination of Lacosamide with Retigabine in Experimental Animals: An Isobolographic Analysis. Pharmacology. 2018; 101(1–2): 22–28. doi: 10.1159/000480019.
38.
Riaz T, Abbasi MA, Rehman A, et al. Enzyme inhibitory, antifungal, antibacterial and hemolytic potential of various fractions of Colebrookia oppositifolia. Pak J Pharm Sci. 2017; 30(1): 105–112.
39.
Linder KA, Kauffman CA, Patel TS, et al. Evaluation of targeted versus universal prophylaxis for the prevention of invasive fungal infections following lung transplantation. Transpl Infect Dis. 2020: e13448. doi: 10.1111/t id.134 48.
40.
Kim JH, Cheng LW, Chan KL, et al. Antifungal Drug Repurposing. Antibiotics (Basel). 2020; 9(11): 812. doi: 10.3390/antibiotics9110812.
41.
Sun N, Li D, Zhang Y, et al. Repurposing an inhibitor of ribosomal biogenesis with broad anti-fungal activity. Sci Rep. 2017; 7(1): 17014. doi: 10.1038/s41598-017-17147-x.
42.
Wu J, Ni T, Chai X, et al. Molecular docking, design, synthesis and antifungal activity study of novel triazole derivatives. Eur J Med Chem. 2018; 143: 1840–1846. doi: 10.1016/j.ejmech.2017.10.081.
43.
Pekmezovic M, Aleksic I, Barac A, et al. Prevention of polymicrobial biofilms composed of Pseudomonas aeruginosa and pathogenic fungi by essential oils from selected Citrus species. Pathog Dis. 2016; 74(8): ft w102. doi: 10.1093/femspd/ft w102.
44.
Denkova-Kostova R, Teneva D, Tomova T, et al. Chemical composition, antioxidant and antimicrobial activity of essential oils from tangerine (Citrus reticulata L.), grapefruit (Citrus paradisi L.), lemon (Citrus lemon L.) and cinnamon (Cinnamomum zeylanicum Blume). Z Naturforsch C J Biosci. 2020; 76(5–6): 175–185. doi: 10.1515/znc-2020-0126.
45.
Císarová M, Tančinová D, Medo J, Kačániová M. The in vitro effect of selected essential oils on the growth and mycotoxin production of Aspergillus species. J Environ Sci Health B. 2016; 51(10): 668–674. doi: 10.1080/03601234.2016.1191887.
46.
Zehetner P, Höferl M, Buchbauer G. Essential oil components and cytochrome P450 enzymes: a review. Flavour and Fragrance Journal. 2019; 34(4): 223–240. doi: https://doi.org/10.1002/ffj.34....
47.
Alammari AH, Shoieb SM, Maayah ZH, El-Kadi AOS. Fluconazole Represses Cytochrome P450 1B1 and Its Associated Arachidonic Acid Metabolites in the Heart and Protects Against Angiotensin II-Induced Cardiac Hypertrophy. J Pharm Sci. 2020; 109(7): 2321–2335. doi: 10.1016/j.xphs.2020.03.016.
48.
Masuda M, Watanabe S, Tanaka M, et al. Screening of furanocoumarin derivatives as cytochrome P450 3A4 inhibitors in citrus. J Clin Pharm Ther. 2018; 43(1): 15–20. doi: 10.1111/jcpt.12595.
49.
OuYang Q, Tao N, Jing G. Transcriptional profiling analysis of Penicillium digitatum, the causal agent of citrus green mold, unravels an inhibited ergosterol biosynthesis pathway in response to citral. BMC Genomics. 2016; 17(1): 599. doi: 10.1186/s12864-016-2943-4.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.