Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Pathophysiology of ischemic reperfusion injury and the molecular targets involved in amelioration of brain injury by herbal medicine
1
Bayero University, Kano, Nigeria
J Pre Clin Clin Res. 2021;15(2):87-99
KEYWORDS
TOPICS
ABSTRACT
Introduction and objectives:
A number of preclinical evaluations of stroke treatment with herbal medicine (HM) have been reported. The aim of the current review was to highlight the pathophysiology of stroke and review the pre-clinically identified molecular mechanisms of HM treatment.
Material and Methods:
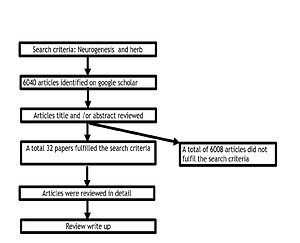
Only 32 articles published in the English language were accessible on Google scholar describing the treatment and mechanistic processes of HM in animal models of stroke, as well as human clinical trials, and were reviewed in this study.
Results and discussion:
Suboptimal Na+/K+ ATPases pump activity, actions of microglia cytokines that increase the level intracellular adhesion molecules-1 (ICAM-1) which promote WBC extravasation with associated increased in matrix metalloproteinase (MMP) activity (digest basement-membranes), explains edema and apoptosis/inflammation. Altered conductivity in injured neurons with compensatory increase in glutamate release that overwhelms the regulatory glial glutamate transporter 1, and thus peaks the level of glutamate to an excitotoxin leve, promotes neuronal death. Glutamate activity on NMDAR promotes oxidative stress, lipid peroxidation and release/influx of Ca2+ that causes apoptosis. The molecular targets involved in the treatment for stroke by HM promote anti-apoptotic/anti-inflammation, anti-oxidation, angiogenesis, neurogenesis, anticoagulation/fibrinolysis effects and optimal metabolism. Different HM promotes the activities of tissue plasminogen activator, haemeoxygenase 1, Neutrin-1, brain derived neurotropic factor (BDNF) and mitogen-activated protein kinase (MAPK).
Conclusions:
The pathophysiology of stroke and the preclinical targets on which HM act to ameliorate them were identified which could serve as a focus for research on the development of effective treatment for stroke.
A number of preclinical evaluations of stroke treatment with herbal medicine (HM) have been reported. The aim of the current review was to highlight the pathophysiology of stroke and review the pre-clinically identified molecular mechanisms of HM treatment.
Material and Methods:
Only 32 articles published in the English language were accessible on Google scholar describing the treatment and mechanistic processes of HM in animal models of stroke, as well as human clinical trials, and were reviewed in this study.
Results and discussion:
Suboptimal Na+/K+ ATPases pump activity, actions of microglia cytokines that increase the level intracellular adhesion molecules-1 (ICAM-1) which promote WBC extravasation with associated increased in matrix metalloproteinase (MMP) activity (digest basement-membranes), explains edema and apoptosis/inflammation. Altered conductivity in injured neurons with compensatory increase in glutamate release that overwhelms the regulatory glial glutamate transporter 1, and thus peaks the level of glutamate to an excitotoxin leve, promotes neuronal death. Glutamate activity on NMDAR promotes oxidative stress, lipid peroxidation and release/influx of Ca2+ that causes apoptosis. The molecular targets involved in the treatment for stroke by HM promote anti-apoptotic/anti-inflammation, anti-oxidation, angiogenesis, neurogenesis, anticoagulation/fibrinolysis effects and optimal metabolism. Different HM promotes the activities of tissue plasminogen activator, haemeoxygenase 1, Neutrin-1, brain derived neurotropic factor (BDNF) and mitogen-activated protein kinase (MAPK).
Conclusions:
The pathophysiology of stroke and the preclinical targets on which HM act to ameliorate them were identified which could serve as a focus for research on the development of effective treatment for stroke.
Ibrahim Mohammed Badamasi. Pathophysiology of ischemic reperfusion injury and the molecular targets involved in amelioration of brain 9 injury by herbal medicine.J Pre-Clin Clin Res. 2021; 15(2): 87–99. doi: 10.26444/jpccr/134526
REFERENCES (140)
1.
Del Zoppo GJ. The neurovascular unit in the setting of stroke. J Intern Med. 2010; 267(2): 156–71. doi: 10.1111/j.1365-2796.2009.02199.x.
2.
Woodruff TM, Thundyil J, Tang SC, Sobey CG, Taylor SM, Arumugam TV. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol Neurodegener. 2011; 6(1): 1–19. doi: 10.1186/1750-1326-6-11.
3.
Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs. 2010; 11(3): 298–308.
4.
Tian C, Cao X, Wang J. Recanalisation therapy in patients with acute ischaemic stroke caused by large artery occlusion: Choice of therapeutic strategy according to underlying aetiological mechanism? Stroke Vasc Neurol. 2017; 2(4): 244–50. doi: 10.1136/svn-2017-000090.
5.
Haghjoo R, Tadjalli M. Effect of Persian Sage (Salvia rhytidia) Extract on Histomorphometric Changes of Cerebral Cortex and Hippocampus Following Ischemia-Reperfusion Injuries in Rat. Zahedan J Res Med Sci. 2016; 18(2): e5993. doi: 10.17795/zjrms-5993.
6.
McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007; 27: 4403–12. doi: 10.1523/JNEUROSCI.5376-06.2007.
7.
Zhou Z, Wei X, Xiang J, Gao J, Wang L, You J, et al. Protection of erythropoietin against ischemic neurovascular unit injuries through the effects of connexin43. Biochem Biophys Res Commun. 2015; 458: 656–62. doi: 10.1016/j.bbrc.2015.02.020.
8.
Pan J, Lei X, Wang J, Huang S, Wang Y, Zhang Y, et al. Effects of Kaixinjieyu, a Chinese herbal medicine preparation, on neurovascular unit dysfunction in rats with vascular depression. BMC Complement Altern Med. 2015; 15: 291. doi: 10.1186/s12906-015-0808-z.
9.
Vangilder RL, Rosen CL, Barr TL, Huber JD. Targeting the neurovascular unit for treatment of neurological disorders. Pharmacol Ther. 2011; 130: 239–47. doi: 10.1016/j.pharmthera.2010.12.004.
10.
Niu T, Qin ZS, Xu X, Liu JS. Bayesian Haplotype Inference for Multiple Linked Single-Nucleotide Polymorphisms. Am J Hum Genet. 2002; 70: 157–169. doi: 10.1086/338446.
11.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018; 49: e46–e110. doi: 10.1161/STR.0000000000000158.
12.
Zhang X, Zheng W, Wang T, Ren P, Wang F, Ma X, et al. Danshen-Chuanxiong-Honghua ameliorates cerebral impairment and improves spatial cognitive deficits after transient focal ischemia and identification of active compounds. Front Pharmacol. 2017; 8: 452. doi: 10.3389/fphar.2017.00452.
13.
Montaner J, Molina CA, Monasterio J, Abilleira S, Arenillas JF, Ribó M, et al. Matrix metalloproteinase-9 pretreatment level predicts intracranial hemorrhagic complications after thrombolysis in human stroke. Circulation. 2003; 107: 598–603. doi: 10.1161/01.CIR.0000046451.38849.90.
14.
Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004; 35: 2659–61. doi: 10.1161/01.STR.0000144051.32131.09.
15.
Wang Y, Fan X, Qu H, Gao X, Cheng Y. Strategies and Techniques for Multi-Component Drug Design from Medicinal Herbs and Traditional Chinese Medicine. Curr Top Med Chem. 2012; 12: 1356–1362. doi: 10.2174/156802612801319034.
16.
Zheng X-W, Shan C-S, Xu Q-Q, Wang Y, Shi Y-H, Wang Y, et al. Buyang Huanwu Decoction Targets SIRT1/VEGF Pathway to Promote Angiogenesis After Cerebral Ischemia/Reperfusion Injury. Front Neurosci. 2018; 12: 911. doi: 10.3389/fnins.2018.00911.
17.
Darioush S. O, Reza. R, Mazyar H, Homayoun S-B, Mohsen S-B, Elyar S-H, Sina Z, Ehsan S. The effect of Ginkgo biloba on functional outcome of patients with acute ischemic stroke: A double-blind, placebo-controlled, randomized clinical trial. J Stroke Cerebrovasc Dis. 2013; 22: e557–563. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.010.
18.
Xia W, Lu YJ, Yuan DC, Kong DZ, Qi WC KF. Efficacy of Huatuo Zaizao Pills for ischemic stroke. Eval Anal Drug Hosp China. 2012; 12: 772–5. doi: 10.1177/0265407510389126.
19.
Cai YF, Xu Y, Guo JW, Zhang XC, Li WF, Liang WX HY. The meta-analysis about the clinical efficacy of Huatuo Zaizao pills on ischemic stroke. Chin Tradit Herb Drug. 2007; 4: 581–4. doi: 10.1111/j.1537-4726.2004.133_10.x.
20.
Castro P, Azevedo E, Serrador J, Rocha I, Sorond F. Hemorrhagic transformation and cerebral edema in acute ischemic stroke: Link to cerebral autoregulation. J Neurol Sci. 2017; 372: 256–61. doi: 10.1016/j.jns.2016.11.065.
21.
Jiang M, Li J, Peng Q, Liu Y, Liu W, Luo C, et al. Neuroprotective effects of bilobalide on cerebral ischemia and reperfusion injury are associated with inhibition of pro-inflammatory mediator production and down-regulation of JNK1/2 and p38 MAPK activation. J Neuroinflammation. 2014; 11(1): 167. doi: 10.1186/s12974-014-0167-6.
22.
Pillai DR, Dittmar MS, Baldaranov D, Heidemann RM, Henning EC, Schuierer G, et al. Cerebral ischemia-reperfusion injury in rats – A 3 T MRI study on biphasic blood-brain barrier opening and the dynamics of edema formation. J Cereb Blood Flow Metab. 2009; 29(11): 1846–55. doi: 10.1038/jcbfm.2009.106.
23.
Oh TW, Park KH, Jung HW, Park YK. Neuroprotective effect of the hairy root extract of Angelica gigas NAKAI on transient focal cerebral ischemia in rats through tOh, T. W, Park KH, Jung HW, Park YK. Neuroprotective effect of the hairy root extract of Angelica gigas NA. BMC Complement Altern Med. 2015; 15: 101. doi: 10.1186/s12906-015-0589-4.
24.
Sawada N, Murata M, Kikuchi K, Osanai M, Tobioka H, Kojima T, et al. Tight junctions and human diseases. Med Electron Microsc. 2003; 36: 147–56. doi: 10.1007/s00795-003-0219-y.
25.
Stevenson BR. Understanding tight junction clinical physiology at the molecular level. J Clin Invest. 1999; 104: 3–4. doi: 10.1172/JCI7599.
26.
Furuse M, Itoh M, Hirase T, Nagaftichi A, Yonemura S, Tsukita S, et al. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J Cell Biol. 1994; 127: 1617–26. doi: 10.1083/jcb.127.6.1617.
27.
Bardutzky J, Schwab S. Antiedema therapy in ischemic stroke. Stroke. 2007; 38(11): 3084–94. doi: 10.1161/STROKEAHA.107.490193.
28.
Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003; 4: 399–415. doi: 10.1038/nrn1106.
29.
Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science (80-). 1999; 286(5449): 2511–4. doi: 10.1126/science.286.5449.2511.
30.
Barrington J, Lemarchand E, Allan SM. A brain in flame; do inflammasomes and pyroptosis influence stroke pathology? Brain Pathol. 2017; 27: 205–212. doi: 10.1111/bpa.12476.
31.
Ramsauer M, Krause D, Dermietzel R. Angiogenesis of the blood-brain barrier in vitro and the function of cerebral pericytes. FASEB J. 2002; 16: 1274–6. doi: 10.1096/fj.01-0814fje.
32.
Fruttiger M, Calver AR, Krüger WH, Mudhar HS, Michalovich D, Takakura N, et al. PDGF mediates a neuron-astrocyte interaction in the developing retina. Neuron. 1996; 17: 1117–31. doi: 10.1016/S0896-6273(00)80244-5.
33.
Shan H, Bian Y, Shu Z, Zhang L, Zhu J, Ding J, et al. Fluoxetine protects against IL-1ß-induced neuronal apoptosis via downregulation of p53. Neuropharmacology. 2016; 107: 68–78. doi: 10.1016/j.neuropharm.2016.03.019.
34.
Nicotera P, Bano D. The Enemy at the Gates: Ca2+ Entry through TRPM7 Channels and Anoxic Neuronal Death. Cell. 2003; 115(7): 768–70. doi: 10.1016/S0092-8674(03)01019-5.
35.
Yeh TH, Hwang HM, Chen JJ, Wu T, Li AH, Wang HL. Glutamate transporter function of rat hippocampal astrocytes is impaired following the global ischemia. Neurobiol Dis. 2005; 18(3): 476–83. doi: 10.1016/j.nbd.2004.12.011.
36.
Rao VLR, Dogan A, Bowen KK, Todd KG, Dempsey RJ. Antisense knockdown of the glial glutamate transporter GLT-1 exacerbates hippocampal neuronal damage following traumatic injury to rat brain. Eur J Neurosci. 2001; 13(1): 119–28. doi: 10.1046/j.1460-9568.2001.01367.x.
37.
Bruhn T, Levy LM, Nielsen M, Christensen T, Johansen FF, Diemer NH. Ischemia induced changes in expression of the astrocyte glutamate transporter GLT1 in hippocampus of the rat. Neurochem Int. 2000; 37(2–3): 277–85. doi: 10.1016/S0197-0186(00)00029-2.
38.
Xuan Chi X, Xu ZC. Potassium currents in CA1 neurons of rat hippocampus increase shortly after transient cerebral ischemia. Neurosci Lett. 2000; 281(1): 5–8. doi: 10.1016/S0304-3940(00)00812-0.
39.
Decollogne S, Bertrand IB, Ascensio M, Drubaix I, Lelievre LG. Na+, K+-ATPase and Na+/Ca2+ exchange isoforms: Physiological and physiopathological relevance. J Cardiovasc Pharmacol. 1993; 22: S96–8.
40.
Simard JM, Tarasov K V, Gerzanich V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim Biophys Acta – Mol Basis Dis. 2007; 1772: 947–57. doi: 10.1016/j.bbadis.2007.03.004.
41.
Salińska E, Lazarewicz JW. NMDA receptor-mediated calcium fluxes in the hippocampus: relevance to ischemic brain pathology. Neurol Neurochir Pol. 1996; 30: 35–42.
42.
Xiong ZG, Chu XP, Simon RP. Ca2+-permeable acid-sensing ion channels and ischemic brain injury. J Membr Biol. 2006; 209: 59–68. doi: 10.1007/s00232-005-0840-x.
43.
Evans PH, Free radicals in brain metabolism and pathology. Br Med Bull. 1993; 49(3): 577–87. doi: 10.1210/er.2006-0040.
44.
Khooei AR, Hosseinzade H IM. Pathologic evaluation of anti-ischemic effect of Salvia leriifolia Benth seed and leaf extracts in rats after global cerebral ischemia. Iran J Basic Med Sci. 2003; 5(4): 8–13. doi: 10.4103/1735-5362.213985 LK -.
45.
Hosseinzadeh H, Hosseini A, Nassiri-Asl M, Sadeghnia HR. Effect of Salvia leriifolia Benth. root extracts on ischemia-reperfusion in rat skeletal muscle. BMC Complement Altern Med. 2007; 7: 23. doi: 10.1186/1472-6882-7-23.
46.
Ke Q, Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006; 70(5): 1469–1480.
47.
Chen Y, Han L, Li J, Chen Y, Zhang M, Qian L, et al. Human urinary kallidinogenase promotes angiogenesis and cerebral perfusion in experimental stroke. PLoS One. 2015; 10(7): e0134543. doi: 10.1371/journal.pone.0134543.
48.
Rosenblum WI. Cytotoxic edema: Monitoring its magnitude and contribution to brain swelling. J Neuropathol Exp Neurol. 2007; 66(9): 771–778. doi.org/10.1097/nen.0b013e3181461965.
49.
Giraldez T, Dominguez J, de la Rosa DA. ENaC in the Brain – Future Perspectives and Pharmacological Implications. Curr Mol Pharmacol. 2016; 6(1): 44–49. doi: 10.2174/1874467211306010006.
50.
Zhou Y, Li HQ, Lu L, Fu DL, Liu AJ, Li JH, et al. Ginsenoside Rg1 provides neuroprotection against blood brain barrier disruption and neurological injury in a rat model of cerebral ischemia/reperfusion through downregulation of aquaporin 4 expression. Phytomedicine. 2014; 21(7): 998–1003. doi.org/10.1016/j.phymed.2013.12.005.
51.
Ruan L, Wang B, Zhuge Q, Jin K. Coupling of neurogenesis and angiogenesis after ischemic stroke. Brain Res. 2015; 1623: 166–173. doi: 10.1016/j.brainres.2015.02.042.
52.
Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R. et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: Both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005; 25(11): 1491–1504. doi: 10.1038/sj.jcbfm.9600148.
53.
Chen ZZ, Gong X, Guo Q, Zhao H, Wang L. Bu Yang Huan Wu decoction prevents reperfusion injury following ischemic stroke in rats via inhibition of HIF-1 ? VEGF and promotion ß-ENaC expression. J Ethnopharmacol. 2019; 10(228): 70–81. doi: 10.1016/j.jep.2018.09.017.
54.
Baranova O, Miranda LF, Pichiule P, Dragatsis I, Johnson RS, Chavez JC. Neuron-specific inactivation of the hypoxia inducible factor 1? increases brain injury in a mouse model of transient focal cerebral ischemia. J Neurosci. 2007; 27(23): 6320–6332. doi: 10.1523/JNEUROSCI.0449-07.2007.
55.
Yeh WL, Lu DY, Lin CJ, Liou HC, Fu WM. Inhibition of hypoxia-induced increase of blood-brain barrier permeability by YC-1 through the antagonism of HIF-1? accumulation and VEGF expression. Molecular Pharmacol. 2007; 72(2): 440–449. doi: 10.1124/mol.107.036418.
56.
Lee JH, Cui HS, Shin SK, Kim JM, Kim SY, Lee JE, et al. Effect of propofol post-treatment on blood-brain barrier integrity and cerebral edema after transient cerebral ischemia in rats. Neurochem Res. 2013; 38(11): 2276–2286. doi: 10.1007/s11064-013-1136-7.
57.
Nada SE, Shah ZA. Preconditioning with Ginkgo biloba (EGb 761®) provides neuroprotection through HO1 and CRMP2. Neurobiol Dis. 2012; 46: 180–189. doi.org/10.1016/j.nbd.2012.01.006.
58.
Nada SE, Tulsulkar J, Shah, ZA. Heme oxygenase 1-mediated neurogenesis is enhanced by Ginkgo biloba (EGb 761®) after permanent ischemic stroke in mice. Molecular Neurobiol. 2014; 49: 945–956. doi: 10.1007/s12035-013-8572-x.
59.
Shah ZA, Nada SE, Doré S. Heme oxygenase 1, beneficial role in permanent ischemic stroke and in Gingko biloba (EGb 761)neuroprotection. Neurosci. 2011; 180: 248–255. doi: 10.1016/j.neuroscience.2011.02.031.
60.
Cisowki J, Loboda A, Jozkowicz A, Chen S, Agarwai A, Dulak J. Role of heme oxygenase-1 in hydrogen peroxide-induced VEGF synthesis: Effect of HO-1 knockout. Biochem Biophys Res Commun. 2005; 326: 670–676. doi: 10.1016/j.bbrc.2004.11.083.
61.
Lin HH, Lai SC, Chau LY. Heme oxygenase-1/carbon monoxide induces vascular endothelial growth factor expression via p38 kinase-dependent activation of Sp1. J Biol Chem. 2011; 286: 3829–3838. doi.org/10.1074/jbc.M110.168831.
62.
Crews L, Ruf R, Patrick C, Dumaop W, Trejo-Morales M, Achim CL, et al. Phosphorylation of collapsin response mediator protein-2 disrupts neuronal maturation in a model of adult neurogenesis: Implications for neurodegenerative disorders. Mol Neurodegener. 2011; 6: 67. doi.org/10.1186/1750-1326-6-67.
63.
Higurashi M, Iketani M, Takei K, Yamashita N, Aoki R, Kawahara N, Goshima Y. Localized role of CRMP1 and CRMP2 in neurite outgrowth and growth cone steering. Dev Neurobiol. 2012; 72: 1528–1540. doi.org/10.1002/dneu.22017.
64.
Ren Z, Li Y, Zhang R, Li Y, Yang Z, Yang H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int J Mol Med. 2017; 40(5): 1444–1456(13). doi: 10.3892/ijmm.2017.3127.
65.
Kim JJ, Khan WI. 5-HT7 receptor signaling: improved therapeutic strategy in gut disorders. Front Behav Neurosci. 2014; 8: 396. doi:10.3389/fnbeh.2014.00396.
66.
Arvidsson A, Collin T, Kirk D, Kokaia Z, Olle L. Neuronal replacement from endogenous precursors in the adult. Nat Med. 2003; 8: 963–970. doi: 10.1038/nm747.
67.
Rueger M, Backes H, Walberer M, Neumaier B, Ullrich R, Emig B, et al. Non-invasive imaging of endogenous neural stem cell mobilization in vivo using Positron Emission Tomography. Klinische Neurophysiol. 2012; 7: 75–83. doi: 10.1523/JNEUROSCI.6092-09.2010.
68.
Carmichael ST. Themes and strategies for studying the biology of stroke recovery in the poststroke epoch. Stroke. 2008; 39: 1380–1388. doi: 10.1161/STROKEAHA.107.499962.
69.
Tang X, Jang SW, Okada M, Chan CB, Feng Y, Liu Y, et al. Netrin-1 mediates neuronal survival through PIKE-L interaction with the dependence receptor UNC5B. Nat Cell Biol. 2008; 10: 698–706. doi: 10.1038/ncb1732.
70.
Nishimoto S, Nishida E. MAPK signalling: ERK5 versus ERK1/2. EMBO Rep. 2006; 7: 782–786. doi: 10.1038/sj.embor.7400755.
71.
Berwick DC, Calissano M, Corness JD, Cook SJ, Latchman DS. Regulation Of Brn-3a N-terminal transcriptional activity by MEK1/2-ERK1/2 signalling in neural differentiation. Brain Res. 2009; 1256: 8–18. doi: 10.1016/j.brainres.2008.12.009.
72.
Huang CY, Chang YM, Kuo WH, Lai TY, Shih YT, Tsai FJ, et al. RSC96 schwann cell proliferation and survival induced by dilong through PI3K/Akt signaling mediated by IGF-I. Evidence-based Complement Altern Med. 2011; 216148. doi: 10.1093/ecam/nep216.
73.
Duan Z, Zhang X, Zhu GX, Gao Y, Xue X. Activation of mGluR4 promotes proliferation of rat neural progenitor cells while mediating activation of ERK1/2 signaling pathway. Cell Mol Biol (Noisy-le-grand). 2013; 59(suppl): OL1809–1817.
74.
Ishii A, Furusho M, Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J Neurosci. 2013; 33: 175–186. doi:10.1523/JNEUROSCI.4403-12.2013.
75.
Jinglong T, Weijuan G, Jun L, Tao Q, Hongbo Z, Shasha L. The molecular and electrophysiological mechanism of Buyanghuanwu Decoction in learning and memory ability of vascular dementia rats. Brain Res Bull. 2013; 99: 13–8. doi: 10.1016/j.brainresbull.2013.09.002.
76.
Han J, Zhang JZ, Zhong ZF, Li ZF, Pang WS, Hu J, et al. Gualou Guizhi decoction promotes neurological functional recovery and neurogenesis following focal cerebral ischemia/reperfusion. Neural Regen Res. 2018; 13(8): 1408–1416. doi: 10.4103/1673-5374.235296.
77.
Stokowska A, Atkins AL, Morán J, Pekny T, Bulmer L, Pascoe MC, et al. Complement peptide C3a stimulates neural plasticity after experimental brain ischaemia. Brain. 2017; 140: 353–369. doi: 10.1093/brain/aww314.
78.
Hou Y jin, Kang H peng. Effects of acupuncture and rehabilitation therapy on the expression of growth associated protein-43 and synaptophysin at the injury site of cerebral palsy rats. Chinese J Tissue Eng Res. 2016; 20: 3999–4005. doi: 10.3969/j.issn.2095-4344.2016.27.007.
79.
Shruster A, Ben-Zur T, Melamed E, Offen D. Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PLoS One. 2012; 7: e40843. doi: 10.1371/journal.pone.0040843.
80.
Raghavan A, Shah ZA. Repair and regeneration properties of Ginkgo biloba after ischemic brain injury. Neural Regen Res. 2014; 9(1): 1104–1107. doi: 10.4103/1673-5374.135308.
81.
Mu Q, Liu P, Hu X, Gao H, Zheng X, Huang H. Neuroprotective ffects of buyang huanwu decoction on cerebral ischemia-induced neuronal damage. Neural Regen Res. 2014; 9(17): 1621–1627. doi: 10.4103/1673-5374.141791.
82.
Duan S, Wang T, Zhang J, Li M, Lu C, Wang L, et al. Huatuo Zaizao pill promotes functional recovery and neurogenesis after cerebral ischemia-reperfusion in rats. BMC Complement Altern Med. 2017; 17: 19. doi: 10.1186/s12906-016-1516-z.
83.
Yamamoto KK, Gonzalez GA, Biggs WH, Montminy MR. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988; 334(6182): 494–8. doi: 10.1038/334494a0.
84.
Nehlig A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br J Clin Pharmacol. 2013; 75(3): 716–27. doi: 10.1111/j.1365-2125.2012.04378.x.
85.
Iguchi H, Mitsui T, Ishida M, Kanba S, Arita J. cAMP response element-binding protein (CREB) is required for epidermal growth factor (EGF)-induced cell proliferation and serum response element activation in neural stem cells isolated from the forebrain subventricular zone of adult mice. Endocr J. 2011; 58(9): 747–59. doi: 10.1507/endocrj.K11E-104.
86.
Wei S, Yin X, Kou Y, Jiang, B. Lumbricus extract promotes the regeneration of injured peripheral nerve in rats. J Ethnopharmacol. 2009; 123: 51–54. doi:10.1016/j.jep.2009.02.030.
87.
Ren Y, Houghton P, Hider RC. Relevant activities of extracts and constituents of animals used in traditional Chinese medicine for central nervous system effects associated with Alzheimer’s disease. J Pharm Pharmacol. 2006; 58(7): 989–96. doi: 10.1211/jpp.58.7.0015.
88.
Zaleska MM, Mercado MLT, Chavez J, Feuerstein GZ, Pangalos MN, Wood A. The development of stroke therapeutics: Promising mechanisms and translational challenges. Neuropharmacol. 2009; 56: 329–341. doi: 10.1016/j.neuropharm.2008.10.006.
89.
Neumann S, Shields NJ, Balle T, Chebib M CA. Innate Immunity and Inflammation Post-Stroke: An alpha7-Nicotinic Agonist Perspective. Int J Mol Sci. 2016; 16: 29029–29046. doi: 10.1111/joa.12473.
90.
Catterall WA. Structure and Regulation of Voltage-Gated Ca 2+ Channels. Annu Rev Cell Dev Biol. 2000; 16: 521–555. doi: 10.1146/annurev.cellbio.16.1.521.
91.
Sun K, Wang CS, Guo J, Horie Y, Fang SP, Wang F, et al. Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharide-induced microcirculatory disturbance in rat mesentery. Life Sci. 2007; 81: 509–518. doi: 10.1016/j.lfs.2007.06.008.
92.
Sun J, Bi Y, Guo L, Qi X, Zhang J, Li G, et al. Buyang Huanwu Decoction promotes growth and differentiation of neural progenitor cells: Using a serum pharmacological method. J Ethnopharmacol. 2007; 113: 199–203. doi: 10.1016/j.jep.2007.05.018.
93.
Wu PF, Zhang Z, Wang F, Chen JG. Natural compounds from traditional medicinal herbs in the treatment of cerebral ischemia/reperfusion injury. Acta Pharmacol Sin. 2010; 31: 1523–31. doi: 10.1038/aps.2010.186.
94.
Gong G, Xiang L, Yuan L, Hu L, Wu W, Cai L, et al. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS One. 2014; 9: e89450. doi: 10.1371/journal.pone.0089450.
95.
Kim DH, Lee HE, Kwon KJ, Park SJ, Heo H, Lee Y, et al. Early immature neuronal death initiates cerebral ischemia-induced neurogenesis in the dentate gyrus. Neuroscience. 2015; 284C: 42–54. doi: 10.1016/j.neuroscience.2014.09.074.
96.
Koushki D, Latifi S, Javidan AN, Matin M. Efficacy of some non-conventional herbal medications (sulforaphane, tanshinone iia, and tetramethylpyrazine) in inducing neuroprotection in comparison with interleukin-10 after spinal cord injury: A meta-analysis. J Spinal Cord Med. 2015; 38(1): 13–22. doi: 10.1179/2045772314Y.0000000215.
97.
Zhang M, Gao F, Teng F, Zhang C. Tetramethylpyrazine promotes the proliferation and migration of brain endothelial cells. Mol Med Rep. 2014; 10: 29–32. doi:10.3892/mmr.2014.2169.
98.
Zhang Q, Zhao YH. Therapeutic angiogenesis after ischemic stroke: Chinese medicines, bone marrow stromal cells (bmscs) and their combinational treatment. Am J Chin Med. 2014; 42(01): 61–77. doi: 10.1142/S0192415X14500049.
99.
Wang HW, Liou KT, Wang YH, Lu CK, Lin YL, Lee IJ, et al. Deciphering the neuroprotective mechanisms of Bu-yang Huan-wu decoction by an integrative neurofunctional and genomic approach in ischemic stroke mice. J Ethnopharmacol. 2011; 138: 22–33. doi: 10.1016/j.jep.2011.06.033.
100.
Oja SS, Saransaari P. Ischemia Induces Release of Endogenous Amino Acids from the Cerebral Cortex and Cerebellum of Developing and Adult Mice. J Amino Acids. 2013; 2013(839036). doi: 10.1155/2013/839036.
101.
Wang L, Huang Y, Wu J, Lv G, Zhou L, Jia J. Effect of Buyang Huanwu decoction on amino acid content in cerebrospinal fluid of rats during ischemic/reperfusion injury. J Pharm Biomed Anal. 2013; 86: 143–150. doi: 10.1016/j.jpba.2013.07.046.
102.
Eweka A, Eweka A, Om’Iniabohs F. Histological studies of the effects of monosodium glutamate on the fallopian tubes of adult female wistar rats. Ann Biomed Sci. 2011; 2: 146–9. doi: 10.4314/abs.v9i1.66569.
103.
Fei X, Zhang X, Wang Q, Li J, Shen H, Wang X, et al. Xijiao dihuang decoction alleviates ischemic brain injury in MCAO rats by regulating inflammation, neurogenesis, and angiogenesis. Evidence-based Complement Altern Med. 2018; 2018(3): 1–12. doi: 10.1155/2018/5945128.
104.
Ahmed ME, Tucker D, Dong Y, Lu Y, Zhao N, Wang R, et al. Methylene Blue promotes cortical neurogenesis and ameliorates behavioral deficit after photothrombotic stroke in rats. Neuroscience. 2016; 336: 39–48. doi: 10.1016/j.neuroscience.2016.08.036.
105.
Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009; 9: 418–428.
106.
Tracey KJ. The inflammatory reflex. Nature. 2002; 420: 853–859. doi: 10.1038/nature01321.
107.
Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005; 6: 775–786. doi: 10.1038/nrn1765.
108.
Cai PY, Bodhit A, Derequito R, Ansari S, Abukhalil F, Thenkabail S, et al. Vagus nerve stimulation in ischemic stroke: Old wine in a new bottle. Front Neurol. 2014; 5: 107. doi: 10.3389/fneur.2014.00107.
109.
Townsend MK, Clish CB, Kraft P, Wu C, Souza AL, Deik AA, et al. Reproducibility of metabolomic profiles among men and women in 2 large cohort studies. Clin Chem. 2013; 59(11): 1657–67. doi: 10.1373/clinchem.2012.199133.
110.
Dasgupta P, Rastogi S, Pillai S, Ordonez-Ercan D, Morris M, Haura E, et al. Nicotine induces cell proliferation by -arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006; 116: 2208–2217. doi: 10.1172/jci28164c1.
111.
Waite KA, Eng C. REVIEW ARTICLE Protean PTEN: Form and Function. Am J Hum Genet. 2002; 70: 829–44. doi: 10.1086/340026.
112.
Koh PO. Ferulic acid attenuates the down-regulation of MEK/ERK/p90RSK signaling pathway in focal cerebral ischemic injury. Neurosci Lett. 2015; 588: 18–23. doi: 10.1016/j.neulet.2014.12.047.
113.
Ji B, Geng P, Liu JG, Shi DZ, Wang YY. Effects of active components extracted from Qixue Bingzhi Recipe on proliferation of vascular smooth muscle cells and expressions of platelet-derived growth factor and its receptor genes. J Chinese Integr Med. 2006; 4(1): 30–4. doi: 10.3736/jcim20060109.
114.
Sheu JR, Kan YC, Hung WC, Ko WC, Yen MH. Mechanisms involved in the antiplatelet activity of tetramethylpyrazine in human platelets. Thromb Res. 1997; 88: 259–70. doi: 10.1016/S0049-3848(97)00253-3.
115.
Kong X, Su X, Zhu J, Wang J, Wan H, Zhong M, et al. Neuroprotective effect of buyang huanwu decoction on rat ischemic/reperfusion brain damage by promoting migration of neural precursor cells. Rejuvenation Res. 2014; 17: 264–75. doi: 10.1089/rej.2013.1468.
116.
Zhang WJ, Wojtaa J, Binder BR. Regulation of the fibrinolytic potential of cultured human umbilical vein endothelial cells: Astragaloside IV downregulates plasminogen activator inhibitor-1 and upregulates tissue-type plasminogen activator expression. J Vasc Res. 1997; 34: 273–80. doi: 10.1159/000159234.
117.
Liu B, Luo C, Zheng Z, Xia Z, Zhang Q, Ke C, et al. Shengui Sansheng San extraction is an angiogenic switch via regulations of AKT/mTOR, ERK1/2 and Notch1 signal pathways after ischemic stroke. Phytomedicine. 2018; 44: 20–31. doi: 10.1016/j.phymed.2018.04.025.
118.
Li LJ, Huang Q, Zhang N, Wang G Bin, Liu YH. miR-376b-5p regulates angiogenesis in cerebral ischemia. Mol Med Rep. 2014; 10: 527–535. doi: 10.3892/mmr.2014.2172.
119.
Li L, Saliba P, Reischl S, Marti HH, Kunze R. Neuronal deficiency of HIF prolyl 4-hydroxylase 2 in mice improves ischemic stroke recovery in an HIF dependent manner. Neurobiol Dis. 2016; 91: 221–3. doi: 10.1016/j.nbd.2016.03.018.
120.
Kume T. Ligand-dependent notch signaling in vascular formation. Adv Exp Med Biol. 2012; 727: 210–222. doi: 10.1007/978-1-4614-0899-4_16.
121.
Cai Z, Zhao B, Deng Y, Shangguan S, Zhou F, Zhou W, et al. Notch signaling in cerebrovascular diseases (Review). Mol Med Rep. 2016; 14(4): 2883–98. doi: 10.3892/mmr.2016.5641.
122.
Sewduth R, Santoro MM. “Decoding” angiogenesis: New facets controlling endothelial cell behavior. Front Physiol. 2016; 7: 306. doi: 10.3389/fphys.2016.00306.
123.
Zhang X, Zhang F, Kong D, Wu X, Lian N, Chen L, et al. Tetra-methyl pyrazine inhibits angiotensin II-induced activation of hepatic stellate cells associated with interference of platelet-derived growth factor ß receptor pathways. FEBS J. 2014; 281: 2754–2768. doi: 10.1111/febs.12818.
124.
Michan S, Sinclair D. Sirtuins in mammals: Insights into their biological function. Biochem J. 2007; 404: 1–13. doi: 10.1042/BJ20070140.
125.
Hu T, Chen Y, Jiang Q, Lin J, Li H, Wang P, et al. Overexpressed eNOS upregulates SIRT1 expression and protects mouse pancreatic ß cells from apoptosis. Exp Ther Med. 2017; 14: 1727–1731. doi: 10.3892/etm.2017.4669.
126.
Lee IC, Ho XY, George SE, Goh CW, Sundaram JR, Pang KKL, et al. Oxidative stress promotes SIRT1 recruitment to the GADD34/PP1? complex to activate its deacetylase function. Cell Death Differ. 2018; 25: 255–267. doi: 10.1038/cdd.2017.152.
127.
Potente M, Ghaeni L, Baldessari D, Mostoslavsky R, Rossig L, Dequiedt F, et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007; 21: 2644–2658. doi: 10.1101/gad.435107.
128.
Dong WP, Li NL, Gao DK, Zhen HN, Zhang X, Li FF. Resveratrol attenuates ischemic brain damage in the delayed phase after stroke and induces messenger RNA and protein express for angiogenic factors. J Vasc Surg. 2008; 48: 709–14. doi: 10.1016/j.jvs.2008.04.007.
129.
Ota H, Eto M, Ogawa S, Iijima K, Akishita M, Ouchi Y. Sirt1/eNOS axis as a potential target against vascular senescence, dysfunction and atherosclerosis. J Atheroscler Thromb. 2010; 17: 431–5. doi: 10.5551/jat.3525.
130.
Al Sabti H. Therapeutic angiogenesis in cardiovascular disease. J Cardiothorac Surg. 2007; 2: 49. doi: 10.1186/1749-8090-2-49.
131.
Chen L, Chen X, Xing S, Zhang J, Li J, Dang C, et al. Tongxinluo attenuates neuronal loss and enhances neurogenesis and angiogenesis in the ipsilateral thalamus and improves neurological outcome after focal cortical infarction in hypertensive rats. Restor Neurol Neurosci. 2014; 52: 533–546. doi: 10.3233/RNN-140403.
132.
Meng Z, Li M, He Q, Jiang S, Zhang X, Xiao J, et al. Ectopic expression of human angiopoietin-1 promotes functional recovery and neurogenesis after focal cerebral ischemia. Neurosci. 2014; 267: 135–146. doi: 10.1016/j.neuroscience.2014.02.036.
133.
Doeppner TR, Kaltwasser B, Kuckelkorn U, Henkelein P, Bretschneider E, Kilic E, et al. Systemic Proteasome Inhibition Induces Sustained Post-stroke Neurological Recovery and Neuroprotection via Mechanisms Involving Reversal of Peripheral Immunosuppression and Preservation of Blood–Brain–Barrier Integrity. Mol Neurobiol. 2016; 53: 6332–6341. doi: 10.1007/s12035-015-9533-3.
134.
Shi S, Xie J, Zhong J, Lin S, Zhang T, Sun K, et al. Effects of low oxygen tension on gene profile of soluble growth factors in co-cultured adipose-derived stromal cells and chondrocytes. Cell Prolif. 2016; 49: 341–351. doi: 10.1111/cpr.12259.
135.
Shi S, Lin S, Li Y, Zhang T, Shao X, Tian T, et al. Effects of tetrahedral DNA nanostructures on autophagy in chondrocytes. Chem Commun. 2018; 54: 1327–1330. doi: 10.1039/c7cc09397g.
136.
Shen J, Zhu Y, Yu H, Fan ZX, Xiao F, Wu P, et al. Buyang Huanwu decoction increases angiopoietin-1 expression and promotes angiogenesis and functional outcome after focal cerebral ischemia. J Zhejiang Univ Sci B. 2014; 15: 272–80. doi: 10.1631/jzus.B1300166.
137.
Choi JK, Dedeoglu A, Jenkins BG. Application of MRS to mouse models of neurodegenerative illness. NMR Biomed. 2007; 20: 216–37. doi: 10.1002/nbm.1145.
138.
Wang HZ, Wang L, Zhang N, Zhang Q, Zhao H, Zhang QX. Houshiheisan compound prescription protects neurovascular units after cerebral ischemia. Neural Regen Res. 2014; 9: 741–8. doi: 10.4103/1673-5374.131580.
139.
Kalkan Oguzhanoglu N, Sözeri Varma G, Karadag F, Tümkaya S, Efe M, Kiroglu Y. Prefrontal cortex neurochemical metabolite levels in major depression and the effects of treatment: An1HMRS study. Turk Psikiyatr Derg. 2014; 25: 75–83.
140.
Zhang J, Zou H, Zhang Q, Wang L, Lei J, Wang Y, et al. Effects of Xiaoshuan enteric-coated capsule on neurovascular functions assessed by quantitative multiparametric MRI in a rat model of permanent cerebral ischemia. BMC Complement Altern Med. 2016; 16: 198. doi:10.1186/s12906-016-1184-z.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.