REVIEW PAPER
Multiple sclerosis and pregnancy- treatment considerations
1
Student’s Scientific Association, Department of Neurology, Medical University, Lublin, Poland
2
Department of Neurology, Medical University, Lublin, Poland
Corresponding author
Maciej Piotr Kamieniak
Student’s Scientific Association, Department of Neurology, Medical University of Lublin, Lublin, Poland
Student’s Scientific Association, Department of Neurology, Medical University of Lublin, Lublin, Poland
J Pre Clin Clin Res. 2020;14(4):126-129
KEYWORDS
TOPICS
ABSTRACT
Multiple sclerosis is a chronic inflammatory disease which, due to the destruction of the fibres of the central nervous system in the process of demyelination, leads to numerous neurological symptoms and progressive disability. The disease is autoimmune, which means that myelin is destroyed by the patient's own cells, caused by improper functionong and regulation of the immune system.
Multiple sclerosis affects more and more people and is therefore a significant clinical problem which, to a large Entent, affects women, especially in childbearing age This presents a big challenge for carrying out pregnancy while continuing the therapy, ensuring the safety of the foetus and simultaneously achieving the best possible therapeutic effect. The decision whether the therapy should be continued or whether it should be eliminatedis usually made according to assessment of the possible gains and losse. Despite the lack of clear indications, there are many studies proving the relative safety of the use of individual but not all the drugs during pregnancy. Pregnancy, however, has a fairly good impact on the development of multiple sclerosis, and that safety considerations, especially those concerning the growing foetus, force a decision to change or completely suspend the therapy. In-depth research on the already available and emerging therapeutic pathways in multiple sclerosis bring hope for increasingly better results in the future in the treatment of pregnant patients with multiple sclerosis.
Kamieniak MP, Wolanin N, Jarosz P, Kobiałka I, Kośmider K, Petit V, Rejdak K. Multiple sclerosis and pregnancy – treatment considerations. J Pre Clin Clin Res. 2020; 14(4): 126–129. doi: 10.26444/jpccr/127671
REFERENCES (31)
1.
INDS Multiple Sclerosis Information Page. National Institute of Neurological Disorders and Stroke. 2015.
2.
Nakahara J, Maeda M, Aiso S, et al. Current concepts in multiple sclerosis: autoimmunity versus oligodendrogliopathy. Clinical Reviews in Allergy & Immunology. 2012;42(1):26-34.
3.
Nazareth TA, Rava AR, Polyakov JL, et al. Relapse prevalence, symptoms, and health care engagement: patient insights from the Multiple Sclerosis in America 2017 survey. Mult Scler Relat Disord. 2018;26:219-234.
4.
Losy J, Bartosik-Psujek H, Członkowska A, et al. Leczenie stwardnienia rozsianego zalecenia Polskiego Towarzystwa Neurologicznego. Pol Przegl Neurol. 2016;12(2):80-95.
5.
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502-1517.
6.
Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurological Sciences: Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2001;22(2):117-139.
7.
Sellner J, Kraus J, Awad A, et al. The increasing incidence and prevalence of female multiple sclerosis--a critical analysis of potential environmental factors. Autoimmun Rev. 2011;10(8):495-502.
9.
MacDonald SC, McEkrath TF, Hernández-Díaz S. Pregnancy Outcomes in Women With Multiple Sclerosis. Am J Epidemiol. 2019;188(1):57-66.
10.
Whitecare CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283(5406):1277-1278.
11.
Confavreux C, Hutchinson M, Hours MM, et al. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. New England Journal of Medicine., 1998;339(5):259-291.
12.
Salemi G, Callari G, Gammino M, et al. The relapse rate of multiple sclerosis changes during pregnancy: a cohort study. Acta Neurol Scand. 2004;110(1):23-26.
13.
Jokubaitis VG, Spelman T, Kalincik T, et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann Neurol. 2016;80(1):89-100.
14.
Voskuhl R, Momtazee C. Pregnancy: Effect on Multiple Sclerosis, Treatment Considerations, and Breastfeeding. Neurotherapeutics. 2017;14(4):974-984.
15.
Smets I, Van Deun L, Bohyn C, et al. Corticosteroids in the management of acute multiple sclerosis exacerbations. Acta Neurol Belg. 2017;117(3):623-633.
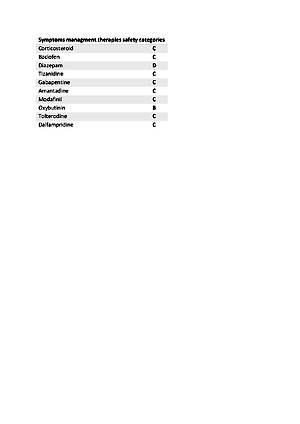
16.
Otero-Romero S, Sastre-Garriga J, Comi G, et al. Pharmacological management of spasticity in multiple sclerosis: Systematic review and consensus paper. Mult Scler. 2016;22(11):1386-1396.
17.
Gold R, Oreja-Guevara C. Advances in the management of multiple sclerosis spasticity: multiple sclerosis spasticity guidelines. Expert Rev Neurother. 2013;13(12 Suppl):55-59.
18.
Generali JA, Cada DJ. Amantadine: multiple sclerosis-related fatigue. Hosp Pharm. 2014;49(8):710-712.
19.
Zonić-Imamović M, Imamović S, Čičkušić A, et al. Effects of Treating an Overactive Urinary Bladder in Patients with Multiple Sclerosis. Acta Med Acad. 2019;48(3):271-277.
20.
Amato MP, Portacio E. Fertility, pregnancy and childbirth in patients with multiple sclerosis: impact of disease-modifying drugs. CNS Drugs. 2015;29(3):207-220.
21.
Levin S, Rimmer K, Vargas WS. Neuroimmunologic disorders in pregnancy. Handb Clin Neurol. 2020;172:105-123.
22.
Batista S, Martins da Silva A, Sá MJ, et al. Recomendações sobre a Abordagem da Esclerose Múltipla na Gravidez, Parto e Pós-Parto: Posição de Consenso do Grupo de Estudos de Esclerose Múltipla [Recommendations about Multiple Sclerosis Management during Pregnancy, Partum and Post-Partum: Consensus Position of the Portuguese Multiple Sclerosis Study Group]. Acta Med Port. 2020;33(9):611-621.
23.
Houtchens MK, Kolb CM. Multiple sclerosis and pregnancy: therapeutic considerations. Journal of Neurology. 2013;260(5):1202-1214.
24.
Thiel S, Langer-Gould A, Rockhoff M, et al. Interferon-beta exposure during first trimester is safe in women with multiple sclerosis-A prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Multiple Sclerosis. 2016;22(6):801-809.
25.
Coyle PK, Sincair SM, Scheuerle AE, et al. Final results from the Betaseron (interferon β-1b) Pregnancy Registry: a prospective observational study of birth defects and pregnancy-related adverse events. BMJ Open, 2014;4(5).
26.
Gold R, Philips JT, Havrdova E, et al. Delayed-Release Dimethyl Fumarate and Pregnancy: Preclinical Studies and Pregnancy Outcomes from Clinical Trials and Postmarketing Experience. Neurol Ther. 2015;4(2):93-104.
27.
Friend S, Richmann S, Bloomgren G, et al. Evaluation of pregnancy outcomes from the Tysabri® (natalizumab) pregnancy exposure registry: a global, observational, follow-up study. BMC Neurol. 2016;16(1):150.
28.
Varytė G, Zakarevičienė J, Ramašauskaitė D, et al. Pregnancy and Multiple Sclerosis: An Update on the Disease Modifying Treatment Strategy and a Review of Pregnancy's Impact on Disease Activity. Medicina (Kaunas). 2020;56(2):49.
29.
Haghikia A, Langer-Gould A, Rellensmann G, et al. Natalizumab use during the third trimester of pregnancy. JAMA Neurol. 2014;71:891-895.
30.
Alroughani R, Altintas A, Al Jumah M, et al. Pregnancy and the Use of Disease-Modifying Therapies in Patients with Multiple Sclerosis: Benefits versus Risks. Multiple Sclerosis International. 2016;2016:.
31.
Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing-remitting multiple sclerosis. Neurol Ther. 2014;3(2):133-138.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.