Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
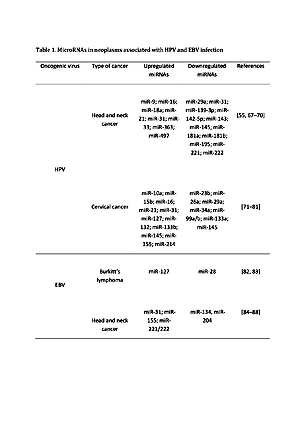
The role of microRNA (miRNA) as a biomarker in HPV and EBV-related cancers
1
Medical University, Lublin, Poland
J Pre Clin Clin Res. 2021;15(2):104-110
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Biomarkers are measurable biological indicators of many disease states. Particularly noteworthy are short nucleotide sequences involved in the regulation of many cellular processes. Their level in body fluids constitutes an important biological marker of serious diseases, such as cancer or cardiovascular diseases. For example, different types of microRNA may be used as biomarker in virus-associated cancers. The aim of this article was to review the current knowledge on the miRNAs and their role in viral-related cancers (EBV and HPV). The article reviews information available in journals and on electronic databases.
Brief description of the state of knowledge:
A significant part of the world’s population hosts at least one of the oncoviruses, but only a small percentage of them undergo a cancerogenesis to which these infectious agents contribute. Interaction between the host cell and viral factors can lead to the origination of a microenvironment favourable to oncogenesis. Cancer arises as a result of dysregulation in many cellular processes, and particularly important are short RNA sequences which regulate the processes that can cause this disease. The varied expression of this ribonucleic acid contributes to many diseases and provides valuable information about health. Importantly, these molecules are differentially expressed in virally-induced cancer. Many publications have confirmed the relationship between the expression of specific types of miRNA and cancers associated with EBV and HPV.
Conclusions:
The use of miRNAs as biomarkers of neoplastic diseases associated with EBV and HPV infections may significantly contribute to the reduction of mortality caused by these viruses, and thanks to the development of modern technologies they are an attractive research object
Biomarkers are measurable biological indicators of many disease states. Particularly noteworthy are short nucleotide sequences involved in the regulation of many cellular processes. Their level in body fluids constitutes an important biological marker of serious diseases, such as cancer or cardiovascular diseases. For example, different types of microRNA may be used as biomarker in virus-associated cancers. The aim of this article was to review the current knowledge on the miRNAs and their role in viral-related cancers (EBV and HPV). The article reviews information available in journals and on electronic databases.
Brief description of the state of knowledge:
A significant part of the world’s population hosts at least one of the oncoviruses, but only a small percentage of them undergo a cancerogenesis to which these infectious agents contribute. Interaction between the host cell and viral factors can lead to the origination of a microenvironment favourable to oncogenesis. Cancer arises as a result of dysregulation in many cellular processes, and particularly important are short RNA sequences which regulate the processes that can cause this disease. The varied expression of this ribonucleic acid contributes to many diseases and provides valuable information about health. Importantly, these molecules are differentially expressed in virally-induced cancer. Many publications have confirmed the relationship between the expression of specific types of miRNA and cancers associated with EBV and HPV.
Conclusions:
The use of miRNAs as biomarkers of neoplastic diseases associated with EBV and HPV infections may significantly contribute to the reduction of mortality caused by these viruses, and thanks to the development of modern technologies they are an attractive research object
Koleśnik M, Stępień E, Polz-Dacewicz M. The role of microRNA (miRNA) as a biomarker in HPV and EBV-related cancers. J Pre-Clin Clin Res. 2021; 15(2): 104–110. doi: 10.26444/jpccr/138306
REFERENCES (110)
1.
Hu B, Niu X, Cheng L, et al. Discovering cancer biomarkers from clinical samples by protein microarrays. PROTEOMICS – Clin Appl. 2015; 9(1–2): 98–110. doi: https://doi.org/10.1002/prca.2....
2.
Romano G, Veneziano D, Acunzo M, Croce CM. Small non-coding RNA and cancer. Carcinogenesis. 2017; 38(5): 485–491. doi: 10.1093/carcin/bgx026.
3.
Hassanpour SH, Dehghani M. Review of cancer from perspective of molecular. J Cancer Res Pract. 2017; 4(4): 127–129. doi: 10.1016/j.j c r pr. 2 017. 0 7. 0 01.
4.
Negrini M, Nicoloso MS, Calin GA. MicroRNAs and cancer—new paradigms in molecular oncology. Curr Opin Cell Biol. 2009; 21(3): 470–479. doi: 10.1016/j.ceb.2009.03.002.
5.
Danarto R, Astuti I, Department of Pharmacology, Universitas Gadjah Mada School of Medicine, Yogyakarta, Indonesia, et al. Urine miR-21–5p and miR-200c-3p as potential non-invasive biomarkers in patients with prostate cancer. Türk Ürol DergisiTurkish J Urol. 2020; 46(1): 26 –30. doi: 10.5152/tud.2019.19163.
6.
Koppers-Lalic D, Hackenberg M, Menezes R de, et al. Non-invasive prostate cancer detection by measuring miRNA variants (isomiRs) in urine extracellular vesicles. Oncotarget. 2016; 7(16): 22566–22578. doi: 10.18632/oncotarget.8124.
7.
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993; 75(5): 843–854. doi: 10.1016/0092-8674(93)90529-Y.
8.
Reinhart BJ, Slack FJ, Basson M, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000; 403(6772): 901–906. doi: 10.1038/35002607.
9.
Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993; 75(5): 855–862. doi: 10.1016/0092-8674(93)9 0530 - 4.
10.
Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP. MicroRNAs in plants. Genes Dev. 2002; 16(13): 1616–1626. doi: 10.1101/gad.1004402.
11.
Cai Y, Yu X, Hu S, Yu J. A Brief Review on the Mechanisms of miRNA Regulation. Genomics Proteomics Bioinformatics. 2009; 7(4): 147–154. doi: 10.1016/S1672-0229(08)60044-3.
12.
Zhou S, Jin J, Wang J, et al. miRNAS in cardiovascular diseases: potential biomarkers, therapeutic targets and challenges. Acta Pharmacol Sin. 2018; 39(7): 1073–1084. doi: 10.1038/aps.2018.30.
13.
Leigh-Ann M, Paul RM. MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics. 2010; 11(7): 537–561.
14.
Panganiban RP, Wang Y, Howrylak J, et al. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 2016; 137(5): 1423–1432. doi: 10.1016/j.jaci.2016.01.029.
15.
Parikh VN, Park J, Nikolic I, et al. Brief Report: Coordinated Modulation of Circulating miR-21 in HIV, HIV-Associated Pulmonary Arterial Hypertension, and HIV/Hepatitis C Virus Coinfection. JAIDS J Acquir Immune Defic Syndr. 2015; 70(3): 236–241. doi: 10.1097/QAI.0000000000000741.
16.
Schulte C, Karakas M, Zeller T. microRNAs in cardiovascular disease – clinical application. Clin Chem Lab Med CCLM. 2017; 55(5): 687–704. doi: 10.1515/cclm-2016-0576.
17.
Patel VD, Capra JA. Ancient human miRNAs are more likely to have broad functions and disease associations than young miRNAs. BMC Genomics. 2017; 18(1): 672. doi: 10.1186/s12864-017-4073-z.
18.
Migita K, Komori A, Kozuru H, et al. Circulating microRNA Profiles in Patients with Type-1 Autoimmune Hepatitis. PLOS ONE. 2015; 10(11): e0136908. doi: 10.1371/journal.pone.0136908.
19.
Qu K, Zhang X, Lin T, et al. Circulating miRNA-21-5p as a diagnostic biomarker for pancreatic cancer: evidence from comprehensive miRNA expression profiling analysis and clinical validation. Sci Rep. 2017; 7(1): 1692. doi: 10.1038/s41598-017-01904-z.
20.
Condrat CE, Thompson DC, Barbu MG, et al. miRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells. 2020; 9(2). doi: 10.3390/cells9020276.
21.
Rounge TB, Lauritzen M, Langseth H, Enerly E, Lyle R, Gislefoss RE. microRNA Biomarker Discovery and High-Throughput DNA Sequencing Are Possible Using Long-term Archived Serum Samples. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2015; 24(9): 1381–1387. doi: 10.1158/1055-9965.EPI-15-0289.
22.
Kappel A, Backes C, Huang Y, et al. MicroRNA in vitro diagnostics using immunoassay analyzers. Clin Chem. 2015; 61(4): 600–607. doi: 10.1373/clinchem.2014.232165.
23.
Dawidowicz M, Kula A, Mielcarska S, et al. miREIA – an immunoassay method in assessment of microRNA levels in tumour tissue-pilot study. The impact of miR-93-5p, miR-142-5p and IFNγ on PD-L1 level in colorectal cancer. Acta Biochim Pol. Published online 2020. doi: 10.18388/abp.2020_5533.
24.
Iorio MV, Croce CM. microRNA involvement in human cancer. Carcinogenesis. 2012; 33(6): 1126–1133. doi: 10.1093/carcin/bgs140
25.
Ahmed EA, El-Basit SAA, Mohamed MA, Swellam M. Clinical role of MiRNA 29a and MiRNA 335 on breast cancer management: their relevance to MMP2 protein level. Arch Physiol Biochem. 2020; 0(0): 1–8. doi: 10.1080/13813455.2020.1749085.
26.
Wang Q, Ye B, Wang P, Yao F, Zhang C, Yu G. Overview of microRNA-199a Regulation in Cancer. Cancer Manag Res. 2019; 11: 10327–10335. doi: 10.2147/CMAR.S231971.
27.
Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. doi: https://doi.org/10.3322/caac.2....
28.
Calin GA, Dumitru CD, Shimizu M, et al. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci. 2002; 99(24): 15524-15529. doi: 10.1073/pnas.242606799.
29.
Klein U, Lia M, Crespo M, et al. The DLEU2/miR-15a/16-1 cluster controls B cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010; 17(1): 28–40. doi: 10.1016/j.ccr.2009.11.019.
30.
Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci. 2005; 102(39): 13944–13949. doi: 10.1073/pnas.0506654102.
31.
Calin GA, Sevignani C, Dumitru CD, et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc Natl Acad Sci USA. 2004; 101(9): 2999–3004. doi: 10.1073/pnas.0307323101.
32.
Sannigrahi MK, Sharma R, Singh V, Panda NK, Rattan V, Khullar M. DNA methylation regulated microRNAs in HPV-16-induced head and neck squamous cell carcinoma (HNSCC). Mol Cell Biochem. 2018; 448(1): 321–333. doi: 10.1007/s11010-018-3336-6.
33.
Saito Y, Liang G, Egger G, et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell. 2006; 9(6): 435–443. doi: 10.1016/j.ccr.2006.04.020.
34.
Tang JT, Wang JL, Du W, et al. MicroRNA 345, a methylation-sensitive microRNA is involved in cell proliferation and invasion in human colorectal cancer. Carcinogenesis. 2011; 32(8): 1207–1215. doi: 10.1093/carcin/bgr114.
35.
Raveche ES, Salerno E, Scaglione BJ, et al. Abnormal microRNA-16 locus with synteny to human 13q14 linked to CLL in NZB mice. Blood. 2007; 109(12): 5079–5086. doi: 10.1182/blood-2007-02-071225.
36.
Hu Z, Chen J, Tian T, et al. Genetic variants of miRNA sequences and non-small cell lung cancer survival. J Clin Invest. 2008; 118(7): 2600–2608. doi: 10.1172/JCI34934.
37.
Aqeilan RI, Calin GA, Croce CM. miR-15a and miR-16–1 in cancer: discovery, function and future perspectives. Cell Death Differ. 2010; 17(2): 215–220. doi: 10.1038/cdd.2009.69.
38.
Calin GA, Ferracin M, Cimmino A, et al. A MicroRNA Signature Associated with Prognosis and Progression in Chronic Lymphocytic Leukemia. N Engl J Med. 2005; 353(17): 1793–1801. doi: 10.1056/NEJMoa050995.
39.
Mui UN, Haley CT, Tyring SK. Viral Oncology: Molecular Biology and Pathogenesis. J Clin Med. 2017; 6(12): 111. doi: 10.3390/jcm6120111.
40.
Chawla JPS, Iyer N, Soodan KS, Sharma A, Khurana SK, Priyadarshni P. Role of miRNA in cancer diagnosis, prognosis, therapy and regulation of its expression by Epstein–Barr virus and human papillomaviruses: With special reference to oral cancer. Oral Oncol. 2015; 51(8): 731–737. doi: 10.1016/j.oraloncolog y.2015.05.008.
41.
Shannon-Lowe C, Rickinson A. The Global Landscape of EBV-Associated Tumours. Front Oncol. 2019; 9. doi: 10.3389/fonc.2019.00713.
42.
Berti FCB, Salviano-Silva A, Beckert HC, de Oliveira KB, Cipolla GA, Malheiros D. From squamous intraepithelial lesions to cervical cancer: Circulating microRNAs as potential biomarkers in cervical carcinogenesis. Biochim Biophys Acta BBA – Rev Cancer. 2019; 1872(2): 188306. doi: 10.1016/j.bbcan.2019.08.001.
43.
Feng YH, Tsao CJ. Emerging role of microRNA-21 in cancer (Review). Biomed Rep. 2016; 5(4): 395–402. doi: 10.3892/br.2016.747.
44.
Fiorucci G, Chiantore MV, Mangino G, Romeo G. MicroRNAs in virus-induced tumourigenesis and IFN system. Cytokine Growth Factor Rev. 2015; 26(2): 183–194. doi: 10.1016/j.cytogfr.2014.11.002.
45.
Snoek BC, Babion I, Koppers-Lalic D, Pegtel DM, Steenbergen RD. Altered microRNA processing proteins in HPV-induced cancers. Curr Opin Virol. 2019; 39: 23–32. doi: 10.1016/j.coviro.2019.07.002.
46.
Pfeffer S, Zavolan M, Grässer FA, et al. Identification of Virus-Encoded MicroRNAs. Science. 2004; 304(5671): 734–736. doi: 10.1126/science.1096781.
47.
Iizasa H, Kim H, Kartika AV, Kanehiro Y, Yoshiyama H. Role of Viral and Host microRNAs in Immune Regulation of Epstein-Barr Virus-Associated Diseases. Front Immunol. 2020; 11. doi: 10.3389/fimmu.2020.00367.
48.
Koleśnik M, Dworzańska A, Polz-Dacewicz M. Wirus Epsteina-Barr w wybranych chorobach nowotworowych. Postępy Biochem. 2020; 66(4): 385–389. doi: 10.18388/pb.2020_364.
49.
Imig J, Motsch N, Zhu JY, et al. microRNA profiling in Epstein–Barr virus-associated B-cell lymphoma. Nucleic Acids Res. 2011; 39(5): 1880–1893. doi: 10.1093/nar/gkq1043.
50.
Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010; 467(7311): 86–90. doi: 10.1038/nature09284.
51.
Rosato P, Anastasiadou E, Garg N, et al. Differential regulation of miR-21 and miR-146a by Epstein–Barr virus-encoded EBNA2. Leukemia. 2012; 26(11): 2343–2352. doi: 10.1038/leu.2012.108.
52.
Jones K, Nourse JP, Keane C, Bhatnagar A, Gandhi MK. Plasma microRNA are disease response biomarkers in classical Hodgkin lymphoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2014; 20(1): 253–264. doi: 10.1158/1078-0432.CCR-13-1024.
53.
Takeuchi T, Kawasaki H, Luce A, et al. Insight toward the MicroRNA Profiling of Laryngeal Cancers: Biological Role and Clinical Impact. Int J Mol Sci. 2020; 21(10): 3693. doi: 10.3390/ijms21103693.
54.
Chang SS, Jiang WW, Smith I, et al. MicroRNA alterations in head and neck squamous cell carcinoma. Int J Cancer. 2008; 123(12): 2791–2797. doi: 10.1002/ijc.23831.
55.
Lajer CB, Garnæs E, Friis-Hansen L, et al. The role of miRNAs in human papilloma virus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Br J Cancer. 2012; 106(9): 1526 –1534. doi: 10.1038/bjc.2012.109.
56.
Park S, Eom K, Kim J, et al. MiR-9, miR-21, and miR-155 as potential biomarkers for HPV positive and negative cervical cancer. BMC Cancer. 2017; 17(1): 658. doi: 10.1186/s12885-017-3642-5.
57.
Estêvão D, Costa NR, Gil da Costa RM, Medeiros R. Hallmarks of HPV carcinogenesis: The role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim Biophys Acta BBA – Gene Regul Mech. 2019; 1862(2): 153–162. doi: 10.1016/j.bbagrm.2019.01.001.
58.
Gupta S, Kumar P, Das BC. HPV: Molecular pathways and targets. Curr Probl Cancer. 2018; 42(2): 161–174. doi:10.1016/j.currproblcancer.2018.03.003.
59.
Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010; 39(4): 493–506. doi: 10.1016/j.molcel.2010.07.023.
60.
Zheng ZM, Wang X. Regulation of cellular miRNA expression by human papillomaviruses. Biochim Biophys Acta. 2011; 1809(11–12): 668– 677. doi: 10.1016/j.bbagrm.2011.05.005.
61.
Wang X, Wang HK, Li Y, et al. microRNAs are biomarkers of oncogenic human papillomavirus infections. Proc Natl Acad Sci. 2014; 111(11): 4262–4267. doi: 10.1073/pnas.1401430111.
62.
Wongjampa W, Ekalaksananan T, Chopjitt P, et al. Suppression of miR-22, a tumour suppressor in cervical cancer, by human papillomavirus 16 E6 via a p53/miR-22/HDAC6 pathway. PloS One. 2018; 13(10): e0206644. doi: 10.1371/journal.pone.0206644.
63.
Pinatti LM, Walline HM, Carey TE. Human Papillomavirus Genome Integration and Head and Neck Cancer. J Dent Res. 2018; 97(6): 691–700. doi: 10.1177/0022034517744213
64.
Chang Y, Moore PS, Weiss RA. Human oncogenic viruses: nature and discovery. Philos Trans R Soc B Biol Sci. 2017; 372(1732): 201602.doi: 10.1098/rstb.2016.0264.
65.
Božinović K, Sabol I, Dediol E, et al. Genome-wide miRNA profiling reinforces the importance of miR-9 in human papillomavirus associated oral and oropharyngeal head and neck cancer. Sci Rep. 2019; 9(1): 2306. doi: 10.1038/s41598-019-38797-z.
66.
Bersani C, Mints M, Tertipis N, et al. MicroRNA-155, -185 and -193b as biomarkers in human papillomavirus positive and negative tonsillar and base of tongue squamous cell carcinoma. Oral Oncol. 2018; 82: 8–16. doi: 10.1016/j.oraloncolog y.2018.04.021.
67.
Lu E, Su J, Zeng W, Zhang C. Enhanced miR-9 promotes laryngo-carcinoma cell survival via down-regulating PTEN. Biomed Pharma-cother Biomedecine Pharmacother. 2016; 84: 608–613. doi: 10.1016/j.biopha.2016.09.047.
68.
Liu M, Wu H, Liu T, et al. Regulation of the cell cycle gene, BTG2, by miR-21 in human laryngeal carcinoma. Cell Res. 2009; 19(7): 828–837. doi: 10.1038/cr.2009.72.
69.
Wald AI, Hoskins EE, Wells SI, Ferris RL, Khan SA. Alteration of microRNA profiles in squamous cell carcinoma of the head and neck cell lines by human papillomavirus. Head Neck. 2011; 33(4): 504–512. doi: 10.1002/hed.21475.
70.
Bufalino A, Cervigne NK, de Oliveira CE, et al. Low miR-143/miR-145 Cluster Levels Induce Activin A Overexpression in Oral Squamous Cell Carcinomas, Which Contributes to Poor Prognosis. PloS One. 2015; 10(8): e0136599. doi: 10.1371/journal.pone.0136599.
71.
Wang N, Zhou Y, Zheng L, Li H. MiR-31 is an independent prognostic factor and functions as an oncomir in cervical cancer via targeting ARID1A. Gynecol Oncol. 2014; 134(1): 129–137. doi: 10.1016/j.ygyno.2014.04.047.
72.
Long MJ, Wu FX, Li P, Liu M, Li X, Tang H. MicroRNA-10a targets CHL1 and promotes cell growth, migration and invasion in human cervical cancer cells. Cancer Lett. 2012; 324(2): 186–196. doi: 10.1016/j.canlet.2012.05.022.
73.
Wang X, Tang S, Le SY, et al. Aberrant Expression of Oncogenic and Tumour-Suppressive MicroRNAs in Cervical Cancer Is Required for Cancer Cell Growth. PLOS ONE. 2008; 3(7): e2557. doi: 10.1371/journal.pone.0002557.
74.
You W, Wang Y, Zheng J. Plasma miR-127 and miR-218 Might Serve as Potential Biomarkers for Cervical Cancer. Reprod Sci. 2015; 22(8): 1037–1041. doi: 10.1177/1933719115570902.
75.
Campos-Viguri GE, Jiménez-Wences H, Peralta-Zaragoza O, et al. miR-23b as a potential tumour suppressor and its regulation by DNA methylation in cervical cancer. Infect Agent Cancer. 2015; 10(1): 42. doi: 10.1186/s13027-015-0037-6.
76.
Dong J, Sui L, Wang Q, Chen M, Sun H. MicroRNA-26a inhibits cell proliferation and invasion of cervical cancer cells by targeting protein tyrosine phosphatase type IVA 1. Mol Med Rep. 2014; 10(3): 1426–1432. doi: 10.3892/mmr.2014.2335.
77.
Yamamoto N, Kinoshita T, Nohata N, et al. Tumour-suppressive microRNA-29a inhibits cancer cell migration and invasion via targeting HSP47 in cervical squamous cell carcinoma. Int J Oncol. 2013; 43(6): 1855–1863. doi: 10.3892/ijo.2013.2145.
78.
Geng D, Song X, Ning F, Song Q, Yin H. MiR-34a Inhibits Viability and Invasion of Human Papillomavirus-Positive Cervical Cancer Cells by Targeting E2F3 and Regulating Survivin. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2015; 25(4): 707–713. doi: 10.1097/IGC.0000000000000399.
79.
Wang L, Chang L, Li Z, et al. miR-99a and -99b inhibit cervical cancer cell proliferation and invasion by targeting mTOR signaling pathway. Med Oncol Northwood Lond Engl. 2014; 31(5): 934. doi: 10.1007/s12032-014-0934-3.
80.
Song X, Shi B, Huang K, Zhang W. miR-133a inhibits cervical cancer growth by targeting EGFR. Oncol Rep. 2015; 34(3): 1573–1580. doi: 10.3892/or.2015.4101.
81.
Zhou X, Yue Y, Wang R, Gong B, Duan Z. MicroRNA-145 inhibits tumourigenesis and invasion of cervical cancer stem cells. Int J Oncol. 2017; 50(3): 853–862. doi: 10.3892/ijo.2017.3857.
82.
Onnis A, Navari M, Antonicelli G, et al. Epstein-Barr nuclear antigen 1 induces expression of the cellular microRNA hsa-miR-127 and impairing B-cell differentiation in EBV-infected memory B cells. New insights into the pathogenesis of Burkitt lymphoma. Blood Cancer J. 2012; 2: e84. doi: 10.1038/bcj.2012.29.
83.
Bartolomé-Izquierdo N, de Yébenes VG, Álvarez-Prado AF, et al. miR-28 regulates the germinal center reaction and blocks tumour growth in preclinical models of non-Hodgkin lymphoma. Blood. 2017; 129(17): 2408–2419. doi: 10.1182/blood-2016-08-731166.
84.
Ni YH, Huang XF, Wang ZY, et al. Upregulation of a potential prognostic biomarker, miR-155, enhances cell proliferation in patients with oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014; 117(2): 227–233. doi: 10.1016/j.oooo.2013.10.017.
85.
Yang CJ, Shen WG, Liu CJ, et al. miR-221 and miR-222 expression increased the growth and tumourigenesis of oral carcinoma cells. J Oral Pathol Med. 2011; 40(7): 560–566. doi: https://doi.org/10.1111/j.1600....
86.
Salazar C, Nagadia R, Pandit P, et al. A novel saliva-based microRNA biomarker panel to detect head and neck cancers. Cell Oncol Dordr. 2014; 37(5): 331–338. doi: 10.1007/s13402-014-0188-2.
87.
Lucas Grzelczyk W, Szemraj J, Kwiatkowska S, Józefowicz-Korczyńska M. Serum expression of selected miRNAs in patients with laryngeal squamous cell carcinoma (LSCC). Diagn Pathol. 2019; 14(1): 49. doi: 10.1186/s13000-019-0823-3.
88.
Yu CC, Chen PN, Peng CY, Yu CH, Chou MY. Suppression of miR-204 enables oral squamous cell carcinomas to promote cancer stemness, EMT traits, and lymph node metastasis. Oncotarget. 2016; 7(15): 20180–20192. doi: 10.18632/oncotarget.7745.
89.
Falzone L, Lupo G, La Rosa GRM, et al. Identification of Novel MicroRNAs and Their Diagnostic and Prognostic Significance in Oral Cancer. Cancers. 2019; 11(5): 610. doi: 10.3390/cancers11050610.
90.
Akbulut N, Oztas B, Kursun S, Evirgen S. Delayed diagnosis of oral squamous cell carcinoma: a case series. J Med Case Reports. 2011; 5(1): 291. doi: 10.1186/1752-1947-5-291.
91.
Zheng J, Sun L, Yuan W, et al. Clinical value of Naa10p and CEA levels in saliva and serum for diagnosis of oral squamous cell carcinoma. J Oral Pathol Med Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2018; 47(9): 830–835. doi: 10.1111/jop.12767.
92.
Yuan C, Yang K, Tang H, Chen D. Diagnostic values of serum tumour markers Cyfra21–1, SCCAg, ferritin, CEA, CA19–9, and AFP in oral/oropharyngeal squamous cell carcinoma. OncoTargets Ther. 2016; 9: 3381–3386. doi: 10.2147/OTT.S105672.
93.
Falzone L, Romano GL, Salemi R, et al. Prognostic significance of deregulated microRNAs in uveal melanomas. Mol Med Rep. 2019; 19(4): 2599–2610. doi: 10.3892/mmr.2019.9949.
94.
Fu X, Han Y, Wu Y, et al. Prognostic role of microRNA-21 in various carcinomas: a systematic review and meta-analysis. Eur J Clin Invest. 2011; 41(11): 1245 –1253. doi: 10.1111/j.1365-2362 .2011.02535.x.
95.
Thorland EC, Myers SL, Gostout BS, Smith DI. Common fragile sites are preferential targets for HPV16 integrations in cervical tumours. Oncogene. 2003; 22(8): 1225–1237. doi: 10.1038/sj.onc.1206170.
96.
Pereira CM, Sehnem D, da Fonseca EO, et al. miRNAs: Important Targets for Oral Cancer Pain Research. BioMed Res Int. 2017; 2017: e4043516. doi: 10.1155/2017/4043516.
97.
Tachibana H, Sho R, Takeda Y, et al. Circulating miR-223 in Oral Cancer: Its Potential as a Novel Diagnostic Biomarker and Therapeutic Target. PLoS ONE. 2016; 11(7). doi: 10.1371/journal.pone.0159693.
98.
Yan ZY, Luo ZQ, Zhang LJ, Li J, Liu JQ. Integrated Analysis and MicroRNA Expression Profiling Identified Seven miRNAs Associated With Progression of Oral Squamous Cell Carcinoma. J Cell Physiol. 2017; 232(8): 2178–2185. doi: 10.1002/jcp.25728.
99.
Chamorro Petronacci CM, Pérez-Sayáns M, Padín Iruegas ME, et al. miRNAs expression of oral squamous cell carcinoma patients: Validation of two putative biomarkers. Medicine (Baltimore). 2019; 98(13): e14922. doi: 10.1097/MD.0000000000014922.
100.
Wu XJ, Pu XM, Zhao ZF, et al. The expression profiles of microRNAs in Kaposi’s sarcoma. Tumour Biol. 2015; 36(1): 437–446. doi: 10.1007/s13277-014-2626-1.
101.
Kim H, Iizasa H, Kanehiro Y, Fekadu S, Yoshiyama H. Herpesviral microRNAs in Cellular Metabolism and Immune Responses. Front Microbiol. 2017; 8. doi: 10.3389/fmicb.2017.01318.
102.
Pratt ZL, Kuzembayeva M, Sengupta S, Sugden B. The microRNAs of Epstein-Barr Virus are expressed at dramatically differing levels among cell lines. Virology. 2009; 386(2): 387–397. doi: 10.1016/j.virol.2009.01.006.
103.
Hassani A, Khan G. Epstein-Barr Virus and miRNAs: Partners in Crime in the Pathogenesis of Multiple Sclerosis? Front Immunol. 2019; 10. doi: 10.3389/fimmu.2019.00695.
104.
Vereide DT, Seto E, Chiu Y-F, et al. Epstein–Barr virus maintains lymphomas via its miRNAs. Oncogene. 2014; 33(10): 1258–1264. doi: 10.1038/onc.2013.71.
105.
Fugl A, Andersen CL. Epstein-Barr virus and its association with disease – a review of relevance to general practice. BMC Fam Pract. 2019; 20(1): 62. doi: 10.1186/s12875-019-0954-3.
106.
Lung RW-M, Hau P-M, Yu KH-O, et al. EBV-encoded miRNAs target ATM-mediated response in nasopharyngeal carcinoma. J Pathol. 2018; 244(4): 394–407. doi: https://doi.org/10.1002/path.5....
107.
Wan X-X, Yi H, Qu J-Q, He Q-Y, Xiao Z-Q. Integrated analysis of the differential cellular and EBV miRNA expression profiles in microdissected nasopharyngeal carcinoma and non-cancerous nasopharyngeal tissues. Oncol Rep. 2015; 34(5): 2585–2601. doi: 10.3892/or.2015.4237.
108.
Cai L, Ye Y, Jiang Q, et al. Epstein–Barr virus-encoded microRNA BART1 induces tumour metastasis by regulating PTEN-dependent pathways in nasopharyngeal carcinoma. Nat Commun. 2015; 6(1): 7353. doi: 10.1038/ncomms8353.
109.
Chan JYW, Gao W, Ho WK, Wei WI, Wong TS. Overexpression of Epstein-Barr virus-encoded microRNA-BART7 in undifferentiated nasopharyngeal carcinoma. Anticancer Res. 2012; 32(8): 3201–3210.
110.
Chen SJ, Chen GH, Chen YH, et al. Characterization of Epstein-Barr Virus miRNAome in Nasopharyngeal Carcinoma by Deep Sequencing. PLOS ONE. 2010; 5(9): e12745. doi: 10.1371/journal.pone.0012745.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.