Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Impact of CCR5-inhibitors on cancer treatment: A systematic review of preclinical evidence
1
Graduate Programme in Biological Sciences, Federal University, Alfenas (UNIFAL-MG), Brazil
2
Department of Oral Diagnosis, School of Dentistry, University of Campinas (UNICAMP), Brazil
3
Laboratory of Molecular Biology and Cell Culture (LBMCC), Faculty of Medicine of Jundiaí (FMJ), Brazil
4
Graduate Programme in Health Sciences, Faculty of Medicine of Jundiaí (FMJ), Brazil
5
Departmentof Oral Diagnosis, School of Dentistry, University of Campinas (UNICAMP), Brazil
6
Graduate Programme in Oral Biology, School of Dentistry, University of Campinas (UNICAMP), Brazil
7
Departament of Pathology and Parasitology, Federal University of Alfenas (UNIFAL-MG), Brazil
Corresponding author
Carine Ervolino de Oliveira
Departament of Pathology and Parasitology, Federal University of Alfenas (UNIFAL-MG), Rua Gabriel Monteiro da Silva, 700, 37130-000, Alfenas, Brazil
Departament of Pathology and Parasitology, Federal University of Alfenas (UNIFAL-MG), Rua Gabriel Monteiro da Silva, 700, 37130-000, Alfenas, Brazil
J Pre Clin Clin Res. 2025;19(2):75-85
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
In view of the role of the CCR5 chemokine receptor in tumour development and progression, researchers have investigated the effects of its inhibition in different types of cancer. Although promising results have been reported, the efficacy, safety, and methodological quality of the studies need to be analyzed before their application in clinical practice. The aim of this review is to evaluate the approaches used to inhibit CCR5 and assess its effects on cancer development. Additionally, the methodological quality of preclinical animal studies aree evaluated.
Review methods:
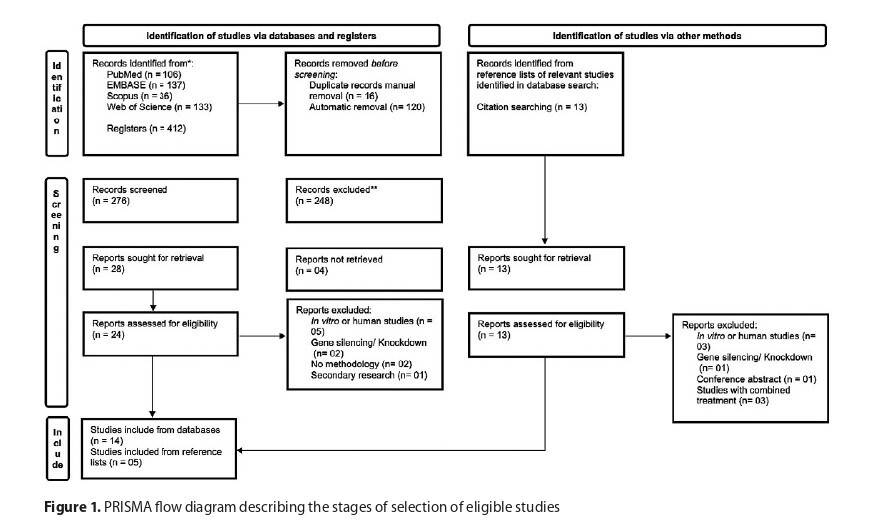
A systematic review was performed according to PRISMA guidelines retrieving, and analyzing 19 original studies. To analyze the risk of bias and quality of the preclinical studies, the SYRCLE tool (Systematic Review Centre for Laboratory Animal Experimentation) was used.
Brief description of the state of knowledge:
Despite the wide methodological variability found in the reviewed studies, some common characteristics were observed. Most experiments (73.68%; n=14) used immunosuppressed mice in their induction models, and the response to CCR5 inhibition was primarily assessed by measuring tumour size. Maraviroc (MVC) was the most frequently used CCR5 inhibitor (73.68%; n=14).
Summary:
The results provided significant evidence that CCR5 inhibition is a promising target for cancer treatment. However, by mapping the risk of bias across all investigated studies, this review provides objective support for guiding future research with more rigorous methodologies, ensuring clear evidence of the impact of CCR5 inhibition on cancer development and progression.
In view of the role of the CCR5 chemokine receptor in tumour development and progression, researchers have investigated the effects of its inhibition in different types of cancer. Although promising results have been reported, the efficacy, safety, and methodological quality of the studies need to be analyzed before their application in clinical practice. The aim of this review is to evaluate the approaches used to inhibit CCR5 and assess its effects on cancer development. Additionally, the methodological quality of preclinical animal studies aree evaluated.
Review methods:
A systematic review was performed according to PRISMA guidelines retrieving, and analyzing 19 original studies. To analyze the risk of bias and quality of the preclinical studies, the SYRCLE tool (Systematic Review Centre for Laboratory Animal Experimentation) was used.
Brief description of the state of knowledge:
Despite the wide methodological variability found in the reviewed studies, some common characteristics were observed. Most experiments (73.68%; n=14) used immunosuppressed mice in their induction models, and the response to CCR5 inhibition was primarily assessed by measuring tumour size. Maraviroc (MVC) was the most frequently used CCR5 inhibitor (73.68%; n=14).
Summary:
The results provided significant evidence that CCR5 inhibition is a promising target for cancer treatment. However, by mapping the risk of bias across all investigated studies, this review provides objective support for guiding future research with more rigorous methodologies, ensuring clear evidence of the impact of CCR5 inhibition on cancer development and progression.
FUNDING
Fundação de Amparo à Pesquisa do Estado de Minas Gerais – FAPEMIG, Minas Gerais, Brazil (APQ-00319-18), Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, Brasília, Brazil (421895/2018-7), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES.
João Lucas Corrêa de Andrade, Bruno Augusto Linhares Almeida Mariz, Nilva Karla Cervigne Furlan, Ricardo D. Coletta, Rômulo Dias Novaes,
Carine Ervolino de Oliveira.Impact of CCR5-inhibitors on cancer treatment – a systematic review of preclinical evidence. J Pre-Clin Clin Res.
2025;19(2):75–85. doi:10.26444/jpccr/205567
REFERENCES (37)
1.
Morein D, Erlichman N, Ben-Baruch A. Beyond Cell Motility: The Expanding Roles of Chemokines and Their Receptors in Malignancy. Front Immunol. 2020;11:952. https://doi.org/10.3389/fimmu.....
2.
Mempel TR, Lill JK, Altenburger LM. How chemokines organize the tumour microenvironment. Nat Rev Cancer. 2024;24(1):28–50. https://doi.org/10.1038/s41568....
3.
Ozga AJ, Chow MT, Luster AD. Chemokines and the immune response to cancer. Immunity. 2021;54(5):859–874. https://doi.org/10.1016/j.immu....
4.
Arnatt CK, Zaidi SA, Zhang Z, et al. Design, syntheses, and characterization of pharmacophore based chemokine receptor CCR5 antagonists as anti prostate cancer agents. Eur J Med Chem. 2013;69:647–658. https://doi.org/10.1016/j.ejme....
5.
Jiao X, Velasco-Velázquez MA, Wang M, et al. CCR5 governs DNA damage repair and breast cancer stem cell expansion. Cancer Res. 2018;78(7):1657–1671. https://doi.org/10.1158/0008-5....
6.
Velasco-Velázquez M, Jiao X, De La Fuente M, et al. CCR5 antagonist blocks metastasis of basal breast cancer cells. Cancer Res. 2012;72(15):3839–3850. https://doi.org/10.1158/0008-5....
7.
Zhang F, Arnatt CK, Haney KM, et al. Structure activity relationship studies of natural product chemokine receptor CCR5 antagonist anibamine toward the development of novel anti prostate cancer agents. Eur J Med Chem. 2012;55:395–408. https://doi.org/10.1016/j.ejme....
8.
Oppermann M. Chemokine receptor CCR5: Insights into structure, function, and regulation. Cell Signal. 2004;16(11):1201–1210. https://doi.org/10.1016/j.cell....
9.
Velasco-Velázquez M, Xolalpa W, Pestell RG. The potential to target CCL5/CCR5 in breast cancer. Expert Opin Ther Targets. 2014;18(11):1265–1275. https://doi.org/10.1517/147282....
10.
Aldinucci D, Borghese C, Casagrande N. The ccl5/ccr5 axis in cancer progression. Cancers (Basel). 2020;12(7):1–30. https://doi.org/10.3390/cancer....
11.
Casagrande N, Borghese C, Visser L, et al. CCR5 antagonism by maraviroc inhibits hodgkin lymphoma microenvironment interactions and xenograft growth. Haematologica. 2019;104(3):564–575. https://doi.org/10.3324/haemat....
12.
Battaglin F, Baca Y, Millstein J, et al. CCR5 and CCL5 gene expression in colorectal cancer: comprehensive profiling and clinical value. J Immunother Cancer. 2024;12(1):e007939. https://doi.org/10.1136/jitc-2....
13.
Domingueti CB, Janini JB, Paranaíba LM, et al. Prognostic value of immunoexpression of CCR4, CCR5, CCR7 and CXCR4 in squamous cell carcinoma of tongue and floor of the mouth. Med Oral Patol Oral Cir Bucal. 2019;24(3):e354-e363. https://doi.org/10.4317/medora....
14.
Singh SK, Mishra MK, Eltoum I-EA, et al. CCR5/CCL5 axis interaction promotes migratory and invasiveness of pancreatic cancer cells. Sci Rep. 2018;8(1):1323. https://doi.org/10.1038/s41598....
15.
Eriksen MB, Frandsen TF. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: a systematic review. J Med Libr Assoc. 2018;106(4):420–431. https://doi.org/10.5195/jmla.2....
16.
Altoé LS, Alves RS, Sarandy MM, et al. Does antibiotic use accelerate or retard cutaneous repair? A systematic review in animal models. Cheungpasitporn W, editor. PLoS One. 2019;14(10):e0223511. https://doi.org/10.1371/journa....
17.
Marcelino RC, Cardoso RM, Domingues ELBC, et al. The emerging risk of microplastics and nanoplastics on the microstructure and function of reproductive organs in mammals: A systematic review of preclinical evidence. Life Sci. 2022;295(February):1–9. https://doi.org/10.1016/j.lfs.....
18.
Ma LL, Wang YY, Yang ZH, et al. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better?. Mil Med Res. 2020;7(1):7. https://doi.org/10.1186/s40779....
19.
Tanabe Y, Sasaki S, Mukaida N, et al. Blockade of the chemokine receptor, CCR5, reduces the growth of orthotopically injected colon cancer cells via limiting cancerassociated fibroblast accumulation. Oncotarget. 2016;7(30):48335–48345. https://doi.org/10.18632/oncot....
20.
Nie Y, Huang HY, Guo MY, et al. Breast Phyllodes Tumours Recruit and Repolarize Tumour – associated macrophages via secreting CCL5 to promote malignant progression, which can be inhibited by CCR5 inhibition therapy. Clin Cancer Res. 2019;25(13):3873–3886. https://doi.org/10.1158/1078-0....
21.
Nishikawa G, Kawada K, Nakagawa J, et al. Bone marrow-derived mesenchymal stem cells promote colorectal cancer progression via CCR5. Cell Death Dis. 2019;10(4):264. https://doi.org/10.1038/s41419....
22.
Zazo S, González-Alonso P, Martín-Aparicio E, et al. Autocrine CCL5 effect mediates trastuzumab resistance by ERK pathway activation in HER2-positive breast cancer. Mol Cancer Ther. 2020;19(8):1696–1707. https://doi.org/10.1158/1535-7....
23.
Wang HC, Chen CW, Yang CL, et al. Tumour-associated macrophages promote epigenetic silencing of gelsolin through DNA methyltransferase 1 in gastric cancer cells. Cancer Immunol Res. 2017;5(10):885–897. https://doi.org/10.1158/2326-6....
24.
Zhou Q, Qi YY, Wang ZW, et al. CCR5 blockade inflames antitumour immunity in BAP1-mutant clear cell renal cell carcinoma. J Immunother Cancer. 2020;8(1). https://doi.org/10.1136/jitc-2....
25.
Ochoa-Callejero L, Pérez-Martínez L, Rubio-Mediavilla S, et al. Maraviroc, a CCR5 antagonist, prevents development of hepatocellular carcinoma in a mouse model. PLoS One. 2013;8(1):e53992. https://doi.org/10.1371/journa....
26.
Mencarelli A, Graziosi L, Renga B, et al. CCR5 antagonism by maraviroc reduces the potential for gastric cancer cell dissemination. Transl Oncol. 2013;6(6):784–793. https://doi.org/10.1593/tlo.13....
27.
Halvorsen EC, Hamilton MJ, Young A, et al. Maraviroc decreases CCL8-mediated migration of CCR5+ regulatory T cells and reduces metastatic tumour growth in the lungs. Oncoimmunology. 2016;5(6):e1150398. https://doi.org/10.1080/216240....
28.
Jiao X, Wang M, Zhang Z, et al. Leronlimab, a humanized monoclonal antibody to CCR5, blocks breast cancer cellular metastasis and enhances cell death induced by DNA damaging chemotherapy. Breast Cancer Res. 2021;23(1):11. https://doi.org/10.1186/s13058....
29.
Pervaiz A, Zepp M, Georges R, et al. Antineoplastic effects of targeting CCR5 and its therapeutic potential for colorectal cancer liver metastasis. J Cancer Res Clin Oncol. 2021;147(1):73–91. https://doi.org/10.1007/s00432....
30.
Sicoli D, Jiao X, Ju X, et al. CCR5 receptor antagonists block metastasis to bone of v-Src oncogene-transformed metastatic prostate cancer cell lines. Cancer Res. 2014;74(23):7103–7114 https://doi.org/10.1158/0008-5....
31.
Tian H, Lyu Y, Yang Y-G, et al. Humanized rodent models for cancer research. Front Oncol. 2020;10(September):1–11. https://doi.org/10.3389/fonc.2....
32.
Sajjad H, Imtiaz S, Noor T, et al. Cancer models in preclinical research: A chronicle review of advancement in effective cancer research. Anim Model Exp Med. 2021;4(2):87–103. https://doi.org/10.1002/ame2.1....
33.
Yang Q. Human cancer xenografts in immunocompromised mice provide an advanced genuine tumour model for research and drug development-A revisit of murine models for human cancers. Biochim Biophys Acta Gen Subj. 2021;1865(8):129929. https://doi.org/10.1016/j.bbag....
34.
Zeng W, Tang Z, Li Y, et al. Patient-derived xenografts of different grade gliomas retain the heterogeneous histological and genetic features of human gliomas. Cancer Cell Int. 2020;20:1. https://doi.org/10.1186/s12935....
35.
Sajjad H, Imtiaz S, Noor T, et al. Cancer models in preclinical research: A chronicle review of advancement in effective cancer research. Animal Model Exp Med. 2021;4(2):87–103. https://doi.org/10.1002/ame2.1....
36.
Weehuizen JM, Wensing AMJ, Mudrikova T, et al. Efficacy and safety of long-term maraviroc use in a heterogeneous group of HIV-infected patients: A retrospective cohort study. Int J Antimicrob Agents. 2019;54(2):215–222. https://doi.org/10.1016/j.ijan....
37.
Patten LW, Blatchford P, Strand M, Kaizer AM. Assessing the performance of different outcomes for tumour growth studies with animal models. Animal Model Exp Med. 2022;5(3):248–257. https://doi.org/10.1002/ame2.1....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.