Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Value of substitute consent and autonomy of research participants in Regulation (EU) No 536/2014
1
Chair and Department of Humanities and Social Medicine, Medical University, Lublin, Poland
Corresponding author
Anna Zagaja
Chair and Department of Humanities and Social Medicine, Medical University, Lublin, Poland
Chair and Department of Humanities and Social Medicine, Medical University, Lublin, Poland
J Pre Clin Clin Res. 2020;14(4):156-159
KEYWORDS
TOPICS
ABSTRACT
Introduction and Objective:
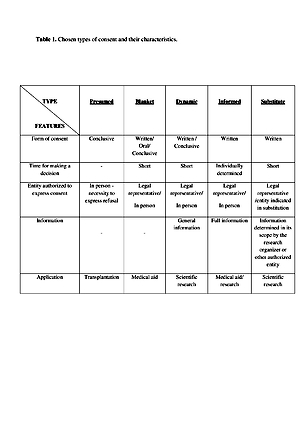
For years the concept of informed consent has been gaining strength as an expression of patient and research participant’s autonomy. The interpreted Regulations allows for obtaining a substitute consent from that person’s legally designated representative which, de facto, is the decision of an authorized entity to include the participant in the study presuming that he/she would agree. The inability to consent in a specific situation and allowing for substitute consent from a different entity (a legally designated representative) is permission to participate in the proposed form of clinical trial. Through the introduced solution, the autonomy of the participant who, for various reasons, cannot consent to participate in clinical trials has been limited. The aim of the study was to analyze the concept of substitute consent in a research setting.
Brief description of the state of knowledge:
The legal obligation to obtain consent originates from the early 20th century. This concept has evolved over the years and broadened in scope to increase the significance of patient autonomy. The analyzed Regulation indicates that the concept of informed consent has been redefined in the clinical research setting to incorporate those who lack the possibility to self-decide. The directive introduces the concept of substitute consent, which is an innovative solution that can affect current research protocols.
Conclusions:
Traditional requirements for informed consent have been omitted (in this redefined Act), which is an innovative legislative solution that can facilitate research
For years the concept of informed consent has been gaining strength as an expression of patient and research participant’s autonomy. The interpreted Regulations allows for obtaining a substitute consent from that person’s legally designated representative which, de facto, is the decision of an authorized entity to include the participant in the study presuming that he/she would agree. The inability to consent in a specific situation and allowing for substitute consent from a different entity (a legally designated representative) is permission to participate in the proposed form of clinical trial. Through the introduced solution, the autonomy of the participant who, for various reasons, cannot consent to participate in clinical trials has been limited. The aim of the study was to analyze the concept of substitute consent in a research setting.
Brief description of the state of knowledge:
The legal obligation to obtain consent originates from the early 20th century. This concept has evolved over the years and broadened in scope to increase the significance of patient autonomy. The analyzed Regulation indicates that the concept of informed consent has been redefined in the clinical research setting to incorporate those who lack the possibility to self-decide. The directive introduces the concept of substitute consent, which is an innovative solution that can affect current research protocols.
Conclusions:
Traditional requirements for informed consent have been omitted (in this redefined Act), which is an innovative legislative solution that can facilitate research
Patryn R, Zagaja A. Value of substitute consent and autonomy of research participants in Regulation (EU) No 536/2014. J Pre-Clin Clin Res. 2020; 14(4): 156–159. doi: 10.26444/jpccr/131221
REFERENCES (28)
1.
Kompanje EJO, Maas AIRm Menon DK, Kesecioglu J, Medical research in emergency research in the European Union member states: tensions between theory and practice. Intensive Care Med. 2014; 40: 496–503.
2.
Millum J, Beecroft B, Hardcastle TC, et alEmergency care research ethics in low-income and middle-income countries. BMJ Global Health.2019; 4: e001260.
3.
El-Menyar A, Asim M, Latifi R, et al. Research in Emergency and Critical Care Settings: Debates, Obstacles and Solutions. Sci Eng Ethics. 2016: 22; 1605–1626. https://doi.org/10.1007/s11948....
4.
Levine AC, Barry AM, Agrawal P, Duber HC, Chang MP, Mackey JM, Hansoti B, Global Health and Emergency Care: Overcoming Clinical Research Barriers. SAEM. 2017; 24, 4: 484–493.
5.
Hurst SA, Vulnerability in research and health care; describing the elephant in the room? Bioethics. 2008; 22: 191–202.
6.
Te nt i E, Simonetti G, Bochicchio M T, Martinelli G, Main changes in European Clinical Trials Regulation (No 536/2014). Contemporary Clinical Trials Communications. 2018; 11; 99–101.
7.
Mende A, Frech M, Riedel C. Principles of the EU Clinical Trials Regulation No 536/2014: What will change? Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz. 2017; 60(8): 795–803.
8.
Mentzelopoulos SD, Mantzanas M, van Belle G, Nichol G, Evolution of European Union legislation on emergency research. Resuscitation. 2015; 91: 84–91.
9.
Saito H, Gill CJ How Frequently Do the Results from Completed US Clinical Trials Enter the Public Domain? – A Statistical Analysis of the ClinicalTrials.gov Database. PLOS ONE. 2014: 9(7): e101826. https://doi.org/10.1371/journa....
10.
Gallin JI, A historical perspectives on clinical research. In: Gallin JI, Ognibene F, editors. Principles and practice of clinical research. Burlington, MA: Elsevier; 2008.
11.
Giannuzzi V, Altavilla A, Ruggieri L, et al. Clinical Trial Application in Europe: What Will Change with the New Regulation? Sci Eng Ethics. 2016; 22: 451.
12.
Rebers S, Aaronson NK, Van Leeuwen FE, Schmidt KM. Exceptions to the rule of informed consent for research with an intervention. BMC Med Ethics. 2016; 6: 17: 9.
13.
Petrini C. Regulation (EU) No 536/2014 on clinical trials on medicinal products for human use: an overview. Ann Ist Super Sanità. 2014; 50(4): 317–321. http://w w w.scielosp.org/pdf/aiss/v50n4/04.pdf.
14.
Farisco M, Evers K, Petrini C. Biomedical research involving patients with disorders of consciousness: ethical and legal dimensions. Ann Ist Super Sanita. 2014; 50(3): 221–8.
15.
Vollmann J, Winau R. Informed consent in human experimentation before the Nurenberg code. BMJ 1996; 313(7070): 1445–1449.
16.
Katz J. Informed consent--a fairy tale? Law’s vision. Univ Pittsbg Law Rev. 1977; 39(2): 137–174.
17.
Beauchamp TL, Informed consent: its history, meaning, and present challenges. Camb Q Healthc Ethics. 2011; 20: 515–523.
18.
Grady Ch. Enduring and Emerging Challenges of Informed Consent. N Engl J Med. 2015; 372: 855–86. doi: 10.1056/NEJMra1411250.
19.
Rozporządzenie Parlamentu Europejskiego i Rady (UE) nr 536/2014, z dnia 16 kwietnia 2014 r. w sprawie badań klinicznych produktów leczniczych stosowanych u ludzi. [4.12.2019]. http://ec.europa.eu/health//si....
20.
Savonitto S, Coppola T, Braglia P, Ciccone A. Informed consent for clinical investigation in the critically ill patient. An introduction to the regulation 536/2014/EC on clinical investigation of medicinal products for human use, repealing Directive 2001/20/EC. GItal Cardiol (Rome). 2016; 17; 326–334.
21.
Rutkowska E. Nowe unijne prawo dotyczące badań klinicznych. [10.06.2020] http://www.rynekzdrowia.pl/Far....
23.
Liddell K, Bion J, Chamberlain D, Druml C, Kompanje E, Lemaire F, Menon D, Vrhovac B, Wiedermann CJ. Medical research involving incapacitated adults: Implications of the EU clinical trials directive 2001/20/EC. Medical Law Review. 2006; 14(3): 367–417.
24.
Karta praw podstawowych Unii Europejskiej 2012/C 326/02 [2.03.2019] http://eur-lex.europa.eu/legal....
25.
Dunn LB, Nowrangi MA, Palmer BW, Jeste DV, Saks ER. Assessing decisional capacity for clinical research or treatment: a review of instruments. Am J Psychiatry. 2006; 163: 1323–34.
26.
Patryn R. Zgoda na wykonanie świadczenia zdrowotnego. Rodzaje zgody, ich zakres i zastosowanie. Neurol. Prakt. 2017; 1: 68–73.
27.
Dal-Ré R, et al. Low risk pragmatic trials do not always require participants’ informed consent. BMJ. 2019; 364(27): l1092. https://doi: 10.1136/bmj.l1092.
28.
Kalkman S, et al. Series: Pragmatic trials and real world evidence: Paper 4. Informed consent. J Clin Epi. 2017; 28: 181–187. https://doi.org/10.1016/j.jcli....
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.