Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Thromboembolic complications in newborns – diagnostic value of D-dimers concentration and proposed outline of enoxaparin use
1
Department of Neonatology and Neonatal Intensive Care Unit, Independent Public Healthcare, Puławy, Poland
2
Department of Veterinary Hygiene, Provincial Veterinary Inspectorate, Lublin, Poland
Corresponding author
Sławomir Jan Wątroba
Department of Neonatology and Neonatal Intensive Care Unit, Independent Public Healthcare, Bema 1, 24-100 Puławy, Poland
Department of Neonatology and Neonatal Intensive Care Unit, Independent Public Healthcare, Bema 1, 24-100 Puławy, Poland
J Pre Clin Clin Res. 2022;16(2):54-64
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
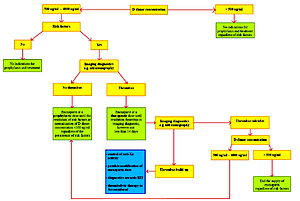
Among paediatric patients, thromboembolic complications (TECs) are most often observed in newborns, especially premature infants requiring intensive care and the use of central vascular accesses. Prognosis depends on the presence of comorbidities, maturity of the newborn, and the location and size of the thrombus. The basic laboratory test that allows for the exclusion of TECs is assessment of the plasma D-dimers concentration, the correct value of which sufficiently excludes the presence of TECs.
Review methods:
The review attempts to systematize existing knowledge on the plasma D-dimers concentration in newborns, and creates a scheme for using enoxaparin (EX), helpful in everyday clinical practice.
Brief description of the state of knowledge:
There are single studies devoted to assessing the plasma D-dimers concentration in newborns, but they agree that the concentration in normal healthy adults does not apply to newborns, regardless of the postmenstrual age (PMA), because the plasma D-dimers concentration found in newborns are significantly higher, despite the lack of clinical and ultrasound features of thrombosis and normal results of other parameters of the coagulation system. Increased plasma D-dimers concentration in newborns may be due to delayed renal clearance of D-dimers and to physiological mechanisms related to the closing of the venous duct (DV) and arterial duct (DA) in the newborn.
Summary:
Plasma D-dimers concentration is one of the basic laboratory markers of TECs, and is a starting point for further diagnostics and a valuable guide when making decisions about prophylactic and therapeutic procedures. The use of EX, as well as other LMWHs, is slowly becoming the treatment of choice in paediatric patients and is increasingly more often recommended in newborns.
Among paediatric patients, thromboembolic complications (TECs) are most often observed in newborns, especially premature infants requiring intensive care and the use of central vascular accesses. Prognosis depends on the presence of comorbidities, maturity of the newborn, and the location and size of the thrombus. The basic laboratory test that allows for the exclusion of TECs is assessment of the plasma D-dimers concentration, the correct value of which sufficiently excludes the presence of TECs.
Review methods:
The review attempts to systematize existing knowledge on the plasma D-dimers concentration in newborns, and creates a scheme for using enoxaparin (EX), helpful in everyday clinical practice.
Brief description of the state of knowledge:
There are single studies devoted to assessing the plasma D-dimers concentration in newborns, but they agree that the concentration in normal healthy adults does not apply to newborns, regardless of the postmenstrual age (PMA), because the plasma D-dimers concentration found in newborns are significantly higher, despite the lack of clinical and ultrasound features of thrombosis and normal results of other parameters of the coagulation system. Increased plasma D-dimers concentration in newborns may be due to delayed renal clearance of D-dimers and to physiological mechanisms related to the closing of the venous duct (DV) and arterial duct (DA) in the newborn.
Summary:
Plasma D-dimers concentration is one of the basic laboratory markers of TECs, and is a starting point for further diagnostics and a valuable guide when making decisions about prophylactic and therapeutic procedures. The use of EX, as well as other LMWHs, is slowly becoming the treatment of choice in paediatric patients and is increasingly more often recommended in newborns.
ABBREVIATIONS
ALP – alkaline phosphatase; ALT – alanin aminotransferase; APTT – activated partial-thromboplastin time; AST – asparaginian aminotransferase; AT-3 – antithrombin-3; BT – bleeding time; CB – conjugated bilirubin; DA – ductus arteriosus; DV –
ductus venosus; ELISA – enzyme-linked immunosorbent assay; EX – enoxaparin; FB – fibrinogen; FPX – fondaparinux; GC – glucocorticoids; GGTP – gamma-glutamyl transpeptidase; Hb – haemoglobin; HCT – haematocrit; HIT – heparininduced
thrombocytopenia; HP – heparin; IMH – intramedullary haemorrhage; INR – international normalized ratio; IUGR – intrauterine growth retardation; LMWH – low-molecular weight heparin; LP – lumbar puncture; NFH – non-fractionated
heparin; OMP – omeprazole; PMA – postmenstrual age; PT – prothrombin time; TB – total bilirubin; TCT – thrombin clotting time; TEC – thromboembolic complication; TEI – thromboembolic incident; TT – thrombolytic treatment
Wątroba SJ, Bryda JR. Thromboembolic complications in newborns – diagnostic value of D-dimers concentration and proposed outline of
enoxaparin use. J Pre-Clin Clin Res. 2022; 16(2): 54–64. doi: 10.26444/jpccr/150257
REFERENCES (110)
1.
Saxonhouse MA. Thrombosis in the neonatal intensive care unit. Clin Perinatol. 2015;42(3):651–673. https://doi.org/10.1016/j.clp.....
2.
Schmidt B, Andrew M. Neonatal thrombosis: report of a prospective Canadian and international registry. Pediatrics. 1995;96(5 Pt 1):939–943.
3.
Will A. Neonatal haemostasis and the management of neonatal thrombosis. Br J Haematol. 2015;169(3):324–332. https://doi.org/10.1111/bjh.13....
4.
Dubbink-Verheij GH, Pelsma ICM, van Ommen CH, et al. Femoral vein catheter is an important risk factor for catheter-related thrombosis in (near-)term neonates. J Pediatr Hematol Oncol. 2018;40(2):e64-e68. https://doi.org/10.1097/MPH.00....
5.
van Ommen CH, Sol JJ. Developmental hemostasis and management of central venous catheter thrombosis in neonates. Semin Thromb Hemost. 2016;42(7):752–759. https://doi.org/10.1055/s-0036....
6.
Toulon P. Developmental hemostasis: laboratory and clinical implications. Int J Lab Hematol. 2016;38(Suppl 1):66–77. https://doi.org/10.1111/ijlh.1....
7.
Tritschler T, Kraaijpoel N, Le Gal G, et al. Venous thromboembolism: advances in diagnosis and treatment. JAMA. 2018;320(15):1583–1594. https://doi.org/10.1001/jama.2....
8.
Zubiatea P, Urrutiaa A, Zamarreño CR, et al. Fiber-based early diagnosis of venous thromboembolic disease by label-free D-dimer detection. Biosens Bioelectron X. 2019;2:100026. https://doi.org/10.1016/j.bios....
9.
Wolberg AS. Fibrinogen and factor XIII: newly recognized roles in venous thrombus formation and composition. Curr Opin Hematol. 2018;25(5):358–364. https://doi.org/10.1097/MOH.00....
10.
Yesudasan S, Wang X, Averett RD. Coarse-grained molecular dynamics simulations of fibrin polymerization: effects of thrombin concentration on fibrin clot structure. J Mol Model. 2018;24(5):109. https://doi.org/10.1007/s00894....
11.
Pieters M, Wolberg AS. Fibrinogen and fibrin: An illustrated review. Res Pract Thromb Haemost. 2019;3(2):161–172. https://doi.org/10.1002/rth2.1....
12.
Crossen J, Diamond SL. Thermal shift assay to probe melting of thrombin, fibrinogen, fibrin monomer, and fibrin: Gly-Pro-Arg-Pro induces a fibrin monomer-like state in fibrinogen. Biochim Biophys Acta Gen Subj. 2021;1865(2):129805. https://doi.org/10.1016/j.bbag....
13.
Piechocka IK, Kurniawan NA, Grimbergen J, et al. Recombinant fibrinogen reveals the differential roles of α- and γ-chain cross-linking and molecular heterogeneity in fibrin clot strain-stiffening. J Thromb Haemost. 2017;15(5):938–949. https://doi.org/10.1111/jth.13....
14.
Brown AC, Baker SR, Douglas AM, et al. Molecular interference of fibrin’s divalent polymerization mechanism enables modulation of multiscale material properties. Biomaterials. 2015;49:27–36. https://doi.org/10.1016/j.biom....
15.
Arora K, Maheshwari N, Sahni G. Design of a thrombin inhibitory staphylokinase based plasminogen activator with anti-reocclusion potential. Int J Biol Macromol. 2020;144:791–800. https://doi. org/10.1016/j.ijbiomac.2019.11.121.
16.
Friedmann AP, Koutychenko A, Wu C, et al. Identification and characterization of a factor Va-binding site on human prothrombin fragment 2. Sci Rep. 2019;9(1):2436. https://doi.org/10.1038/s41598- 019-38857-4.
17.
Giannitsis E, Mair J, Christersson C, et al. How to use D-dimer in acute cardiovascular care. Eur Heart J Acute Cardiovasc Care. 2017;6(1):69–80. https://doi.org/10.1177/204887....
18.
Soomro AY, Guerchicoff A, Nichols DJ, et al. The current role and future prospects of D-dimer biomarker. Eur Heart J Cardiovasc Pharmacother. 2016;2(3):175–184. https://doi.org/10.1093/ehjcvp....
19.
Thachil J, Lippi G, Favaloro EJ. D-Dimer testing: laboratory aspects and current issues. Methods Mol Biol. 2017;1646:91–104. https://doi. org/10.1007/978-1-4939-7196-1_7.
20.
Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D-dimer. J Am Coll Cardiol. 2017;70(19):2411–2420. https://doi.org/10.1016/j. jacc.2017.09.024.
21.
Riley RS, Gilbert AR, Dalton JB, et al. Widely used types and clinical applications of D-dimer assay. Lab Med. 2016;47(2):90–102. https:// doi.org/10.1093/labmed/lmw001.
22.
Favresse J, Lippi G, Roy PM, et al. D-dimer: preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci. 2018;55(8):548–577. https://doi.org/10.1080/104083... 9734.
23.
Jogala S, Rachamalla SS, Aukunuru J. Development of subcutaneous sustained release nanoparticles encapsulating low molecular weight heparin. J Adv Pharm Technol Res. 2015;6(2):58–64. https://doi. org/10.4103/2231-4040.154531.
24.
Starling S. Milestone 8: Targeting the Xa factor. Nat Rev Cardiol. 2017.https://doi.org/10.1038/nrcard....
25.
Okhota S, Melnikov I, Avtaeva Y, et al. Shear stress-induced activation of von willebrand factor and cardiovascular pathology. Int J Mol Sci. 2020;21(20):7804. https://doi.org/10.3390/ijms21....
26.
Miyamoto K, Komatsu H, Nagaya Y, et al. Changes in serum D-dimer level and effect of enoxaparin sodium after a cesarean section: a retrospective study. J Matern Fetal Neonatal Med. 2022;35(3):509–514. https://doi.org/10.1080/147670....
27.
Moffett BS, Lee-Kim Y, Galati M, et al. Population pharmacokinetics of enoxaparin in pediatric patients. Ann Pharmacother. 2018;52(2):140–146. https://doi.org/10.1177/106002....
28.
Qian C, Huhtakangas J, Juvela S, et al. Early vs. late enoxaparin for the prevention of venous thromboembolism in patients with ICH: A double blind placebo controlled multicenter study. Clin Neurol Neurosurg. 2021;202:106534. https://doi.org/10.101 /j.clineuro.2021.106534.
29.
Ankolaa AA, Ghbeisb MB, Bailey B, et al. Utilization practices of low molecular weight heparin in pediatric patients with acquired and congenital heart disease. Prog Pediatr Cardiol. 2021;61:101346. https:// doi.org/10.1016/j.ppedcard.2021.101346.
30.
Murray R, Tobias JT. A case of thrombocytosis associated with enoxaparin therapy in an adolescent. Clin Pharmacol. 2021;13:203–207. https://doi.org/10.2147/CPAA.S....
31.
Haley KM. Neonatal Venous Thromboembolism. Front Pediatr. 2017;5:136. https://doi.org/10.3389/fped.2....
32.
Sirachainan N, Limrungsikul A, Chuansumrit A, et al. Incidences, risk factors and outcomes of neonatal thromboembolism. J Matern Fetal Neonatal Med. 2018;31(3):347–351. https://doi.org/10.1080/147 67058.2017.1285892.
33.
Lassandro G, Palmieri VV, Palladino V, et al. Venous thromboembolism in children: from diagnosis to management. Int J Environ Res Public Health. 2020;17(14):4993. https://doi.org/10.3390/ijerph....
34.
Bhat R, Kwon S, Zaniletti I, et al. Risk factors associated with venous and arterial neonatal thrombosis in the intensive care unit: a multicentre case-control study. Lancet Haematol. 2022;9(3):e200-e207. https://doi. org/10.1016/S2352-3026(21)00399-9.
35.
Mehta JL, Calcaterra G, Bassareo PP. COVID-19, thromboembolic risk, and Virchow’s triad: Lesson from the past. Clin Cardiol. 2020;43(12):1362–1367. https://doi.org/10.1002/clc.23....
36.
Witmer C, Raffini L. Treatment of venous thromboembolism in pediatric patients. Blood. 2020;135(5):335–343. https://doi.org/10.1182/ blood.2019001847.
37.
Garrido-Barbero M, Arnaez J, Loureiro B, et al. The role of factor V leiden, prothrombin G20210A, and MTHFR C677T mutations in neonatal cerebral sinovenous thrombosis. Clin Appl Thromb Hemost. 2019;25:1076029619834352. https://doi.org/10.1177/107602....
38.
Capecchi M, Abbattista M, Martinelli I. Cerebral venous sinus thrombosis. J Thromb Haemost. 2018;16(10):1918–1931. https://doi. org/10.1111/jth.14210.
39.
Hudson IR, Gibson BE, Brownlie J, et al. Increased concentrations of D-dimers in newborn infants. Arch Dis Child. 1990;65(4):383–384. https://doi.org/10.1136/adc.65....
40.
Török-Nagy B, Antal J, Dénes B. Generation and characterization of D-dimer specific monoclonal antibodies for use in latex agglutination test. PLoS One. 2019;14(2):e0212104. https://doi.org/10.1371/journa....
41.
Koltsova EM, Balashova EN, Ignatova AA, et al. Impaired platelet activity and hypercoagulation in healthy term and moderately preterm newborns during the early neonatal period. Pediatr Res. 2019;85(1):63– 71.https://doi.org/10.1038/s41390....
42.
Rodríguez-Peña Y, Ibáñez-Pinilla M. Elevated levels of D-dimer tested by immunoturbidimetry are associated with the extent of severity of pre-eclampsia. Int J Gynaecol Obstet. 2020;150(2):241–247. https:// doi.org/10.1002/ijgo.13163.
43.
Karabay M, Toptan H. Short-term outcomes in neonates and preterm infants with SARS-CoV-2 infection acquired postpartum. J Pediatr Infect Dis. 2021;16(06):290–295. https://doi.org/10.1055/s-0041....
44.
Thomas L. Neonatal hemostasis. In: Kamat D, Frei-Jones M, editors. Benign hematologic disorders in children. Cham, Springe; 2020. p. 335–352.
45.
Heldner MR, Zuurbier SM, Li B, et al. Prediction of cerebral venous thrombosis with a new clinical score and D-dimer levels. Neurology. 2020;95(7):898–909. https://doi.org/10.1212/WNL.00....
46.
Almorad A, Ohanyan A, Pintea Bentea G, et al. D-dimer blood concentrations to exclude left atrial thrombus in patients with atrial fibrillation. Heart. 2021;107(3):195–200. https://doi.org/10.1136/ heartjnl-2020-317612.
47.
Robert-Ebadi H, Robin P, Hugli O, et al. Impact of the ageadjusted D-dimer cutoff to exclude pulmonary embolism: A multinational prospective real-life study (the RELAX-PE Study). Circulation. 2021;143(18):1828–1830. https://doi.org/10.1161/ CIRCULATIONAHA.120.052780.
48.
Tonetti T, Grasselli G, Rucci P, et al. Synergistic effect of static compliance and D-dimers to predict outcome of patients with COVID-19-ARDS: A prospective multicenter study. Biomedicines. 2021;9(9):1228. https:// doi.org/10.3390/biomedicines9091228.
49.
Lu M, Fu M, Zhang Y, et al. Septicaemia with deep venous thrombosis and necrotising pneumonia caused by acute community-acquired methicillin-resistant Staphylococcus aureus in an infant with a threeyear follow-up: a case report. BMC Infect Dis. 2022;22(1):189. https:// doi.org/10.1186/s12879-022-07166-z.
50.
Hochart A, Pierache A, Jeanpierre E, et al. Coagulation standards in healthy newborns and infants. Arch Pediatr. 2021;28(2):156–158. https://doi.org/10.1016/j.arcp....
51.
Chidambaram AG, Kharrubi R, Dugan M. An unusual case of prolonged site bleeding after lumbar puncture in an infant. Pediatrics. 2018;141(1):694. https://doi.org/10.1542/peds.1....
52.
Morrone K. Thrombocytopenia in the newborn. Neoreviews. 2018;19(1):34–41. https://doi.org/10.1542/neo.19....
53.
Cholette JM, Faraoni D, Goobie SM, et al. Patient blood management in pediatric cardiac surgery: a review. Anesth Analg. 2018;127(4):1002–1016. https://doi.org/10.1213/ANE.00....
54.
Monagle P, Newall F. Management of thrombosis in children and neonates: practical use of anticoagulants in children. Hematology Am Soc Hematol Educ Program. 2018;2018(1):399–404. https://doi. org/10.1182/asheducation-2018.1.399.
55.
Marín Gabriel MÁ, Ortiz Movilla R, Muñoz Labián C, et al. [Enoxaparin overdose in a newborn]. Arch Argent Pediatr. 2018;116(6):762–764. https://doi.org/10.5546/aap.20....
56.
Bhat R, Monagle P. Anticoagulation in preterm and term neonates: Why are they special? Thromb Res. 2020;187:113–121. https://doi. org/10.1016/j.thromres.2019.12.019.
57.
Klaassen ILM, Sol JJ, Suijker MH, et al. Are low-molecular-weight heparins safe and effective in children? A systematic review. Blood Rev. 2019;33:33–42. https://doi.org/10.1016/j.blre....
58.
Ting J, Yeung K, Paes B, et al. Thrombosis and hemostasis in newborns (THiN) group. How to use low-molecular-weight heparin to treat neonatal thrombosis in clinical practice. Blood Coagul Fibrinolysis.2021;32(8):531–538. https://doi.org/10.1097/MBC.00....
59.
Rashish G, Paes BA, Nagel K, et al. Thrombosis and hemostasis in newborns (THiN) group. Spontaneous neonatal arterial thromboembolism: infants at risk, diagnosis, treatment, and outcomes. Blood Coagul Fibrinolysis. 2013;24(8):787–797. https://doi.org/10.1097/MBC.b0....
60.
Robinson V, Achey MA, Nag UP, et al. Thrombosis in infants in the neonatal intensive care unit: Analysis of a large national database. J Thromb Haemost. 2021;19(2):400–407. https://doi.org/10.1111/jth.15....
61.
Chen IL, Ou-Yang MC, Chen FS, et al. The equations of the inserted length of percutaneous central venous catheters on neonates in NICU. Pediatr Neonatol. 2019;60(3):305–310. https://doi.org/10.1016/j.pedn....
62.
Bosch A, Albisetti M. Management of venous thromboembolism in children: current recommendations and therapeutic options. Ther Clin Risk Manag. 2020;16:673–679. https://doi.org/10.2147/TCRM.S....
63.
Shi Y, Shi W, Chen L, et al. A systematic review of ultrasound-accelerated catheter-directed thrombolysis in the treatment of deep vein thrombosis. J Thromb Thrombolysis. 2018;45(3):440–451. https://doi.org/10.1007/s11239....
64.
Albisetti M, Schmugge M, Haas R, et al. Arterial thromboembolic complications in critically ill children. J Crit Care. 2005;20(3):296–300. https://doi.org/10.1016/j.jcrc....
65.
Rogberg AN, Gopalan D, Westerlund E, et al. Do radiologists detect chronic thromboembolic disease on computed tomography? Acta Radiol. 2019;60(11):1576–1583. https://doi.org/10.1177/028418....
66.
Ghouri MA, Gupta N, Bhat AP, et al. CT and MR imaging of the upper extremity vasculature: pearls, pitfalls, and challenges. Cardiovasc Diagn Ther. 2019;9(Suppl 1):S152-S173. https://doi.org/10.21037/cdt.2....
67.
Ismail G, Obrișcă B, Jurubiță R, et al. Inherited risk factors of thromboembolic events in patients with primary nephrotic syndrome. Medicina (Kaunas). 2020;56(5):242. https://doi.org/10.3390/medici....
68.
Stolz E, Kaps M, Dorndorf W. Assessment of intracranial venous hemodynamics in normal individuals and patients with cerebral venous thrombosis. Stroke. 1999;30(1):70–75. https://doi.org/10.1161/01.str.... PMID: 9880391.
69.
Lazzareschi I, Curatola A, Gatto A, et al. Diagnosis and management of cerebral venous sinus thrombosis in children: a single-center retrospective analysis. Childs Nerv Syst. 2021;37(1):153–160. https://doi.org/10.1007/s00381....
70.
Abunimer AM, Lak AM, Calvachi P, et al. Early detection and management of venous thrombosis in skull base surgery: role of routine doppler ultrasound monitoring. Neurosurgery. 2022. https://doi.org/10.1227/neu.00....
71.
Kochar PS, Sawhney H, Sharma P, et al. Sonographic diagnosis of neonatal cerebral venous sinus thrombosis. Pediatr Neurol 2020;18(05):236–240. https://doi.org/10.1055/s-0039....
72.
Wu Z, Xie Y, Xiong S, et al. The venous occlusion image score: a novel quantitative scoring instrument for cerebral venous sinus thrombosis. J Stroke Cerebrovasc Dis. 2021;30(7):105845. https://doi.org/10.1016/j.jstr....
73.
Bansod A, Garg RK, Rizvi I, et al. Magnetic resonance venographic findings in patients with tuberculous meningitis: Predictors and outcome. Magn Reson Imaging. 2018;54:8–14. https://doi.org/10.1016/j.mri.....
74.
Niżankowski R, Windyga J. [Deep-vein thrombosis]. In: Szczeklik A, Gajewski P, editors. [Szczeklik’s internal diseases]. Ed. 12. Kraków; 2021. p.554–564.
75.
Streetz VN, Patatanian LK. Intravenous enoxaparin in pediatric burn patients: A case series. J Pediatr Pharmacol Ther. 2019;24(5):456–461. https://doi.org/10.5863/1551-6....
76.
Romantsik O, Bruschettini M, Zappettini S, et al. Heparin for the treatment of thrombosis in neonates. Cochrane Database Syst Rev. 2016;11(11):CD012185. https://doi.org/10.1002/146518....
77.
Goldsmith R, Chan A, Paes B, et al. Feasibility and safety of enoxaparin whole milligram dosing in premature and term neonates. J Perinatol. 2015;35(10):852–854. https://doi.org/10.1038/jp.201....
78.
Molinari AC, Banov L, Bertamino M, et al. A practical approach to the use of low molecular weight heparins in VTE treatment and prophylaxis in children and newborns. Pediatr Hematol Oncol. 2015;32(1):1–10. https://doi.org/10.3109/088800....
79.
Solari F, Varacallo M. Low Molecular Weight Heparin (LMWH). 2022 Feb 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan–. PMID: 30247832.
80.
Nagge J, Crowther M, Hirsh J. Is impaired renal function a contraindication to the use of low-molecular-weight heparin? Arch Intern Med. 2002;162(22):2605–2609. https://doi.org/10.1001/archin....
81.
Pardun N, Lemmer J, Belker K, et al. Low-molecular-weight heparin administered by subcutaneous catheter is a safe and effective anti-coagulation regimen in selected inpatient infants and children with complex congenital heart disease. Cardiol Young. 2021;31(9):1439–1444. https://doi.org/10.1017/S10479....
82.
Wysocki EL, Kuhn A, Steinbrenner J, et al. Enoxaparin dose requirements to achieve therapeutic low-molecular-weight heparin anti-factor Xa levels in infants and young children. J Pediatr Hematol Oncol. 2021;43(7):e946-e950. https://doi.org/10.1097/MPH.00....
83.
Michaels LA, Gurian M, Hegyi T, et al. Low molecular weight heparin in the treatment of venous and arterial thromboses in the premature infant. Pediatrics. 2004;114(3):703–707. https://doi.org/10.1542/peds.2....
84.
Fitchett D. The impact of bleeding in patients with acute coronary syndromes: how to optimize the benefits of treatment and minimize the risk. Can J Cardiol. 2007;23(8):663–671. https://doi.org/10.1016/s0828-....
85.
Al-Taee AM, Ghoulam E, Lee P, et al. Underutilization of peptic ulcer disease prophylaxis among elderly users of antiplatelets and anticoagulants. Dig Dis Sci. 2021;66(10):3476–3481. https://doi.org/10.1007/s10620....
86.
Faure C, Michaud L, Shaghaghi EK, et al. Intravenous omeprazole in children: pharmacokinetics and effect on 24-hour intragastric pH. J Pediatr Gastroenterol Nutr. 2001;33(2):144–148. https://doi.org/10.1097/000051....
87.
Beckmann R, Shaheen H, Kweider N, et al. Enoxaparin prevents steroid-related avascular necrosis of the femoral head. Sci World J. 2014;2014:347813. https://doi.org/10.1155/2014/3....
88.
Kewal KJ. Drug-induced spinal disorders. In: Kewal KJ, editors. Drug-induced neurological disorders. Ed. 4. Switzerland; 2021. p. 511–520. https://doi.org/10.1007/978-3-....
89.
Hwang HG, Koo SM, Uh ST, et al. The perioperative management of antithrombotic therapies using enoxaparin. J Korean Med Sci. 2017;32(6):942–947. https://doi.org/10.3346/jkms.2....
90.
Almeida CR, Francisco EM, Pinho-Oliveira V, et al. Fascia iliaca block associated only with deep sedation in high-risk patients, taking P2Y12 receptor inhibitors, for intramedullary femoral fixation in intertrochanteric hip fracture: a series of 3 cases. J Clin Anesth. 2016;35:339–345. https://doi.org/10.1016/j.jcli....
91.
Eymin G. [Low molecular weight heparin-induced hyperkalemia and hyponatremia. Report of one case]. Rev Med Chil. 2021;149(2):291–294. https://doi.org/10.4067/s0034-....
92.
Custodio M, Thompson EC. Hyperkalemia secondary to prophylactic heparin use in a trauma patient: case report. MJM. 2020;6(3):12. https://doi.org/10.33470/2379-....
93.
Yam L, Bahjri K, Geslani V, et al. Enoxaparin thromboprophylaxis dosing and anti-factor Xa levels in low-weight patients. Pharmacotherapy. 2019;39(7):749–755. https://doi.org/10.1002/phar.2....
94.
Wolsey A, Wilcox RA, Olson JA, et al. Retrospective comparison of two enoxaparin dosing and monitoring protocols at a pediatric hospital. Am J Health Syst Pharm. 2019;76(11):815–819. https://doi.org/10.1093/ajhp/z....
95.
García-Salido A, Antón J, Martínez-Pajares JD, et al. [Spanish consensus document on diagnosis, stabilisation and treatment of pediatric multisystem inflammatory syndrome related to SARS-CoV-2 (SIM-PedS)]. An Pediatr. 2021;94(2):116.e1–116.e11. https://doi.org/10.1016/j.anpe....
96.
Dinh CN, Moffett BS, Galati M, et al. A critical evaluation of enoxaparin dose adjustment guidelines in children. J Pediatr Pharmacol Ther. 2019;24(2):128–133. https://doi.org/10.5863/1551-6....
97.
Wiltrout K, Lissick J, Raschka M, et al. Evaluation of a pediatric enoxaparin dosing protocol and the impact on clinical outcomes. J Pediatr Pharmacol Ther. 2020;25(8):689–696. https://doi.org/10.5863/1551-6....
98.
Kenet G, Barg AA, Nowak-Göttl U. Hemostasis in the very young. Semin Thromb Hemost. 2018;44(7):617–623. https://doi.org/10.1055/s-0038....
99.
Dhakchinamoorthi KK, Palathingal JT, Geethanjali M, et al. Evaluation of factors associated with enoxaparin therapy in south indian patients. Sch Acad J Pharm. 2020;9(10):283–289. https://doi.org/10.36347/sajp.....
100.
Mehershahi S, Mantri N, Kumar A, et al. Enoxaparin-induced liver injury. Case Rep Gastroenterol. 2020;14(2):315–319. https://doi.org/10.1159/000508....
101.
Velasco de Cos G, Sánchez-Molina Acosta I, Iturralde Ros M, et al. Hepatotoxicity with cholestatic pattern secondary to enoxaparin treatment. Adv Lab Med. 2021;2(4):575–578. https://doi.org/10.1515/almed-....
102.
Patriarcheas V, Pikoulas A, Kostis M, et al. Heparin-induced thrombocytopenia: pathophysiology, diagnosis and management. Cureus. 2020;12(3):e7385. https://doi.org/10.7759/cureus....
103.
Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360–3392. https://doi.org/10.1182/blooda....
104.
Fathi M. Heparin-induced thrombocytopenia (HIT): Identification and treatment pathways. Glob Cardiol Sci Pract. 2018;2018(2):15. https://doi.org/10.21542/gcsp.....
105.
Onuoha C, Barton KD, Wong ECC, et al. Therapeutic plasma exchange and intravenous immune globulin in the treatment of heparin-induced thrombocytopenia: A systematic review. Transfusion. 2020;60(11):2714–2736. https://doi.org/10.1111/trf.16....
106.
Lai CMB, Smith T, Lee AYY. Treatment and outcomes of heparin-induced thrombocytopenia (HIT) in patients with neoplasm, a case series. J Thromb Thrombolysis. 2021;51(3):725–733. https://doi.org/10.1007/s11239....
107.
Chok R, Turley E, Bruce A. Screening and diagnosis of heparin-induced thrombocytopenia in the pediatric population: A tertiary centre experience. Thromb Res. 2021;207:1–6. https://doi.org/10.1016/j.thro....
108.
Warkentin TE. Challenges in detecting clinically relevant heparin-induced thrombocytopenia antibodies. Hamostaseologie. 2020;40(4):472–484. https://doi.org/10.1055/a-1223....
109.
Harris EI, Zurbriggen LD, Brunner MJ, et al. Doppler ultrasound screening in patients with newly diagnosed heparin-induced thrombocytopenia. Blood Adv. 2021;5(22):4575–4577. https://doi.org/10.1182/blooda....
110.
Ozturk E, Ayyildiz P, Yildiz O, et al. Fondaparinux treatment in a neonate with heparin induced thrombocytopenia during extracorporeal life support. Maedica (Bucur). 2016;11(1):68–7.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.