Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
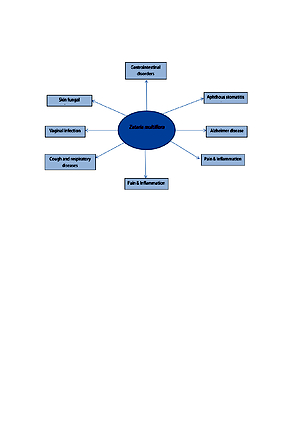
Potential effect and mechanism of action of Zataria multiflora in liver disorders
1
Tabib Daru Company, Kashan, Iran
Corresponding author
Mohaddese Mahboubi
Tabib Daru Company, Second floor, Tabib Building, Bayan 6, Motahari St., 8715143330, Kashan, Iran
Tabib Daru Company, Second floor, Tabib Building, Bayan 6, Motahari St., 8715143330, Kashan, Iran
J Pre Clin Clin Res. 2021;15(4):192-198
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Zataria multiflora Boiss or Iranian thyme (Lamiancea family) is traditionally used for the management of liver disorders. Due to the traditional importance of Z. multiflora in liver diseases, the aim of the review is to evaluate the preventive and therapeutic effects of Z. multiflora in liver diseases.
Review methods:
Information was obtained from original peer reviewed articles published in scientific sources up to September 2021. Google Scholar, PubMed, Scopus, AMED, Cochrane Library electronic literature databases were investigated by the key words of ‘ Zataria multiflora’, ‘liver’, ‘hepato-protective’, ‘ROS’, ‘oxidative stress’, and ‘clinical trial’. The collected data aresummarized, categorized, analyzed, and discussed.
Brief description of the state of knowledge:
The result of one clinical trial showed the efficacy and safety of Z. multiflora powder in non-alcoholic fatty liver diseases. Z. multiflora powder and its extracts reduced the liver oxidative stress induced by drugs, environmental toxins, cyst hydatid, and endogenous factors. The antioxidant activity of Z. multiflora essential oil is higher than its extracts or powder, due to the high content of thymol and carvacrol in its oil. Reactive oxygen species and oxidative stress play important roles in liver diseases; therefore, Z. multiflora and its extracts are regarded as natural antioxidants protecting the liver against free radicals.
Summary:
Although there is one clinical study on Z. multiflora in non-alcoholic fatty liver diseases, large clinical trials are required to evaluate the efficacy and safety of Z. multiflora in liver diseases
Zataria multiflora Boiss or Iranian thyme (Lamiancea family) is traditionally used for the management of liver disorders. Due to the traditional importance of Z. multiflora in liver diseases, the aim of the review is to evaluate the preventive and therapeutic effects of Z. multiflora in liver diseases.
Review methods:
Information was obtained from original peer reviewed articles published in scientific sources up to September 2021. Google Scholar, PubMed, Scopus, AMED, Cochrane Library electronic literature databases were investigated by the key words of ‘ Zataria multiflora’, ‘liver’, ‘hepato-protective’, ‘ROS’, ‘oxidative stress’, and ‘clinical trial’. The collected data aresummarized, categorized, analyzed, and discussed.
Brief description of the state of knowledge:
The result of one clinical trial showed the efficacy and safety of Z. multiflora powder in non-alcoholic fatty liver diseases. Z. multiflora powder and its extracts reduced the liver oxidative stress induced by drugs, environmental toxins, cyst hydatid, and endogenous factors. The antioxidant activity of Z. multiflora essential oil is higher than its extracts or powder, due to the high content of thymol and carvacrol in its oil. Reactive oxygen species and oxidative stress play important roles in liver diseases; therefore, Z. multiflora and its extracts are regarded as natural antioxidants protecting the liver against free radicals.
Summary:
Although there is one clinical study on Z. multiflora in non-alcoholic fatty liver diseases, large clinical trials are required to evaluate the efficacy and safety of Z. multiflora in liver diseases
ABBREVIATIONS
IBS – Irritable bowel syndrome; IBD – Inflammatory bowel disease; ROS – Reactive oxygen species; CAT – catalase; SOD –
super oxide dismutase;GPx – Glutathione peroxidase;GSH – Glutathione; RNS – nitrogen species;ALT – alanine transaminase;
AST – aspartate transaminase;ALP – alkaline phosphatase; HepG2 – human liver cancer cell line;MDA – malondialdehyde;
NAC – N-acetyl cysteine; LDH – lactate dehydrogenase; GST – Glutathione S-transferase; FRAP – ferric reducing ability of
plasma; GGT – Gamma-glutamyl transferase; oH0 – reactive hydroxyl radicals;hs-CRP – high-sensitivity C-reactive protein;
FVC – Forced vital capacity; PEF – peak expiratory flow; HOMA.IR – Homeostatic Model Assessment of Insulin Resistance
Mohaddese Mahboubi. Potential effect and mechanism of action of Zataria multiflora in liver disorders. J Pre-Clin Clin Res. 2021; 15(4):
192–198. doi: 10.26444/jpccr/144424
REFERENCES (67)
1.
Mozaffarian V. Dictionary of Iranian plant names. Tehran, Iran: Farhang-E-Moaser; 1996.
2.
Ali MS, Saleem M, Ali Z, et al. Chemistry of Zataria multiflora (Lamiaceae). Phytochemistry. 2000; 55: 933–936. doi: 10.1016/s0031-9422(00)00249-1.
4.
Bernard F, Hassonpoor H, Shaker-Bazanov H. The effect of carbohydrate type and illumination mode on proline, caffeic acid and rosmarinic acid accumulation in two lines ofZataria multiflora callus tissues. Acta Horticulturae. 2006; 723: 289–292. doi: 10.17660/ActaHortic.2006.723.39.
5.
Aghamohammadi A, Azadbakht M, Hosseinimehr SJ. Quantification of thymol content in different extracts of Zataria multiflora by HPLC method. Journal Pharmaceutical and Biomedical Research. 2016; 2: 8–13. doi: 10.18869/acadpub.pbr.2.1.8.
6.
Shomali T. Chapter 17 – Zataria multiflora and Gastrointestinal Tract Disorders. In: Watson RR, Preedy VR, Hrsg. Dietary Interventions in Gastrointestinal Diseases. Academic Press; 2019: 209–212. doi: https:// doi.org/10.1016/B978-0-12-814468-8.00017-X.
7.
Mahboubi M. Therapeutic Potential of Zataria multiflora Boiss in Treatment of Irritable Bowel Syndrome (IBS). Journal of Dietary Supplements 2019; 16: 119–128. doi: 10.1080/19390211.2017.1409852.
8.
Majlessi N, Choopani S, Kamalinejad M, et al. Amelioration of amyloid β-induced cognitive deficits by Zataria multiflora Boiss. essential oil in a rat model of Alzheimer’s disease. CNS Neurosci Ther. 2012; 18: 295–301. doi: 10.1111/j.1755-5949.2011.00237.x.
9.
Kavoosi G, Balotf S. Zataria multiflora essential oil reduces diabetic damages in streptozotocin-induced diabetic rats. Clinical Biochemistry. 2011; 10: 17632–17639.
10.
Hosseinzadeh H, Ramezani M, Salmani G. Antinociceptive, anti- inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol. 2000; 73: 379–385. doi: 10.1016/s0378-8741(00)00238-5.
11.
Mahboubi M. Management of acute cough byZataria multiflora Boiss as an alternative treatment. J Integr Med. 2018; 16: 20–25. doi: 10.1016/j. joim.2017.12.006.
12.
Mahboubi M. Systematic review: The potency of Zataria multiflora Boiss in treatment of vaginal infections. 2018; 22: 76–83.
13.
Khosravi RA, Shokri H, Farahnejat Z, et al. Antimycotic efficacy of Iranian medicinal plants towards dermatophytes obtained from patients with dermatophytosis. Chinese Journal of Natural Medicines. 2013; 11: 43–48. doi: https://doi.org/10.1016/S1875-....
14.
Mansoori P, Hadji Akhoondi A, Ghavami R, et al. Clinical evaluation of Zataria multiflora essential oil mouthwash in the management of recurrent Aphthous Stomatitis. Daru Journal of Pharmaceutical Sciences. 2002; 10: 74–77.
15.
Diesen DL, Kuo PC. Nitric oxide and redox regulation in the liver: Part I. General considerations and redox biology in hepatitis. J Surg Res. 2010; 162: 95–109. doi: 10.1016/j.jss.2009.09.019.
16.
Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacognosy Reviews. 2010; 4: 118–126. doi: 10.4103/0973-7847.70902.
17.
Saei-Dehkordi SS, Tajik H, Moradi M, et al. Chemical composition of essential oils inZataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food and Chemical Toxicology. 2010; 48: 1562–1567. doi: 10.1016/j.fct.2010.03.025.
18.
Sharififar F, Derakhshanfar A, Dehghan-Nudeh G, et al. In vivo antioxidant activity of Zataria multiflora Boiss essential oil. Pakistan Journal of Pharmaceutical Sciences. 2011; 24: 221–225.
19.
Li S, Tan H-Y, Wang N, et al. The role of oxidative stress and antioxidants in liver diseases. International Journal of Molecular Sciences. 2015; 16: 26087–26124. doi: 10.3390/ijms161125942.
20.
Videla LA. Oxidative stress signaling underlying liver disease and hepatoprotective mechanisms. World J Hepatol. 2009; 1: 72–78. doi: 10.4254/wjh.v1.i1.72.
21.
Mahadevan SB, McKiernan PJ, Davies P, et al. Paracetamol induced hepatotoxicity. Arch Dis Child. 2006; 91: 598–603. doi: 10.1136/ adc.2005.076836.
22.
Sterrenberg L, Julicher RHM, Bast A, et al. Adriamycin stimulates NADPH-dependent lipid peroxidation in liver microsomes not only by enhancing the production of O2 and H2O2, but also by potentiating the catalytic activity of ferrous ions. Toxicology Letters. 1984; 22: 153–159. doi: https://doi.org/10.1016/0378-4....
23.
Ahmed MB, Khater MR. Evaluation of the protective potential of Ambrosia maritima extract on acetaminophen-induced liver damage. Journal of Ethnopharmacology. 2001; 75: 169–174. doi: https://doi. org/10.1016/S0378-8741(00)00400-1.
24.
Ahmadipour A, Sharififar F, Najafi A, et al. Preventive effect of methanolic extract of Zataria Multiflora Boiss on liver toxicity of paracetamol in rats. J Med Life. 2015; 8: 270–274.
25.
Mohebbati R, Paseban M, Soukhtanloo M, et al. Effects of standardized Zataria multiflora extract and its major ingredient, carvacrol, on adriamycin-induced hepatotoxicity in rat. Biomedical Journal. 2018; 41: 340–347. doi: https://doi.org/10.1016/j.bj.2....
26.
Hosseinzadeh H, Ramezani M, Salmani G. Antinociceptive, anti- inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. Journal of ethnopharmacology. 2000; 73: 379–385. doi: 10.1016/s0378-8741(00)00238-5.
27.
Ahani N, Sangtarash MH, Alipour Eskandani M, et al. Zataria multiflora Boiss essential oil induce apoptosis in two human colon cancer cell lines (HCT116 & SW48). Iranian journal of public health. 2020; 49: 753–762.
28.
Piazza MJ, Urbanetz AA. Environmental toxins and the impact of other endocrine disrupting chemicals in women’s reproductive health. JBRA Assisted Reproduction. 2019; 23: 154–164. doi: 10.5935/1518- 0557.20190016.
29.
Ahmadipour A, Sharififar F, Pournamdari M, et al. Hepatoprotective effect of Zataria Multiflora Boiss against malathion-induced oxidative stress in male rats. Oriental Pharmacy and Experimental Medicine. 2016; 16: 287–293. doi: 10.1007/s13596-016-0238-6.
30.
Habibollahi P, Mahboobi N, Esmaeili S, et al. Halothane-induced hepatitis: A forgotten issue in developing countries: Halothane-induced hepatitis. Hepatitis monthly. 2011; 11: 3–6.
31.
Sakhaee E, Emadi L, Azari O, et al. Evaluation of the beneficial effects of Zataria Multiflora Boiss in halothane-induced hepatotoxicity in rats. Advances in Clinical and Experimental Medicine. 2011; 20: 23–29.
32.
Benkerroum N. Chronic and acute toxicities of aflatoxins: Mechanisms of action. Int J Environ Res Public Health. 2020; 17: 423. doi: 10.3390/ ijerph17020423.
33.
Makki OF, Omidi A, Nik HA, et al. Anti-aflatoxin B1 effects of Shirazi thyme (Zataria multiflora) in broilers: evaluation of performance and liver histopathology. 2016; 6: 6090.
34.
Yao Y, Zang Y, Qu J, et al. The toxicity of metallic nanoparticles on liver: The subcellular damages, mechanisms, and outcomes. International Journal of Nanomedicine. 2019; 14: 8787–8804. doi: 10.2147/IJN.S212907.
35.
Kohi NH, Rasooli A, Hajihosseini R, et al. Considering the Zataria multiflora essential oil effect on the xenobiotic metabolism in acute toxicity induced by iron nanoparticle. Journal of Animal Research. 2018; 31: 172–182.
36.
Shokrzadeh M, Chabra A, Ahmadi A, et al. Hepatoprotective effects of Zataria multiflora ethanolic extract on liver toxicity induced by cyclophosphamide in mice. Drug Res. 2015; 65: 169–175. doi: 10.1055/s- 0034-1370932.
37.
Sarwar R, Pierce N, Koppe S. Obesity and nonalcoholic fatty liver disease: current perspectives. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy. 2018; 11: 533–542. doi: 10.2147/DMSO.S146339.
38.
Kavoosi G. Zataria multiflora essential oil reduces diabetic damages in streptozotocin-induced diabetic rats. African Journal of Biotechnology 2011; 10: 17632–17639.
39.
Mahmoodi M, Koohpeyma F, Saki F, et al. The protective effect of Zataria multiflora Boiss. hydroalcoholic extract on TNF-α production, oxidative stress, and insulin level in streptozotocin-induced diabetic rats. Avicenna Journal of Phytomedicine. 2019; 9: 72–83.
40.
Khoshvaghti A, Nazifi S, Derakhshaniyan S, et al. The Effects ofZataria multiflora hydroalcoholic extract on some liver enzymes, cholesterol, triglyceride, HDL-Cholesterol, LDL Cholesterol, Albumin and Total Protein in Rat. J Basic Appl Sci. 2012; 8: 217–222.
41.
Mohammadi A, Gholamhoseinian A, Fallah H. Zataria multiflora increases insulin sensitivity and PPARγ gene expression in high fructose fed insulin resistant rats. Iran J Basic Med Sci. 2014; 17: 263–270.
42.
Pedrosa I, Saíz A, Arrazola J, et al. Hydatid disease: Radiologic and pathologic features and complications. 2000; 20: 795–817. doi: 10.1148/ radiographics.20.3.g00ma06795.
43.
Moazeni M, Larki S, Saharkhiz MJ, et al. In vivo study of the efficacy of the aromatic water of Zataria multiflora on hydatid cysts. Antimicrob Agents Chemother. 2014; 58: 6003–6008. doi: 10.1128/aac.02963-14.
44.
Moazeni M, Larki S, Oryan A, et al. Preventive and therapeutic effects of Zataria multiflora methanolic extract on hydatid cyst: an in vivo study. Vet Parasitol. 2014; 205: 107–112. doi: 10.1016/j.vetpar.2014.07.006.
45.
Oryan A, Moazeni M, Kordshouli F. Administration of Zataria multiflora as a novel therapeutic strategy in destruction of the germinal layer of hydatid cyst. Research Journal of Parasitology. 2016; 11: 41–47. doi: 10.3923/jp.2016.41.47.
46.
Al-abodi HR, Al-Shadeedi SMJ, K.Z.K A-A, et al.Zataria multiflora Bois as an auspicious therapeutic approach againstEchinococcus granulosus: Current status and future perspectives. Comparative Immunology, Microbiology and Infectious Diseases. 2019; 66: 101335. doi: https:// doi.org/10.1016/j.cimid.2019.101335.
47.
Atayi Z, Borji H, Moazeni M, et al. Zataria multiflora would attenuate the hepatotoxicity of long-term albendazole treatment in mice with cystic echinococcosis. Parasitology International. 2018; 67: 184–187. doi: 10.1016/j.parint.2017.11.007.
48.
Zamani N, Shams M, Nimrouzi M, et al. The effects of Zataria multiflora Boiss. (Shirazi thyme) on nonalcoholic fatty liver disease and insulin resistance: A randomized double-blind placebo-controlled clinical trial. Complement Ther Med. 2018; 41: 118–123. doi: 10.1016/j. ctim.2018.09.010.
49.
Khazdair MR, Rajabi O, Balali-Mood M, et al. The effect of Zataria multiflora on pulmonary function tests, hematological and oxidant/ antioxidant parameters in sulfur mustard exposed veterans, a randomized doubled-blind clinical trial. Environ Toxicol Pharmacol. 2018; 58: 180–188. doi: 10.1016/j.etap.2018.01.006.
50.
Nazari A, Mahmazi S, Sahraian Z. Evaluation of Zataria multiflora Boiss. (Shirazi Thyme) hydro alcoholic extract effect on pro-inflammatory cytokine IL-6 different transcript variants expression level in mesenchymal stem cells (MSCs). Gene, Cell and Tissue. 2018; 5: e82463. doi: 10.5812/gct.82463.
51.
Aristatile B, Al-Numair KS, Veeramani C, et al. Effect of carvacrol on hepatic marker enzymes and antioxidant status in D-galactosamine-induced hepatotoxicity in rats. Journal Fundamental Clinical Pharmacology. 2009; 23: 757–765.
52.
Nafees S, Ahmad ST, Arjumand W, et al. Carvacrol ameliorates thioacetamide-induced hepatotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in liver of Wistar rats. Hum Exp Toxicol. 2013; 32: 1292–1304. doi: 10.1177/0960327113499047.
53.
Aristatile B, Al-Numair KS, Veeramani C, et al. Effect of carvacrol on hepatic marker enzymes and antioxidant status in D-galactosamine-induced hepatotoxicity in rats. Fundamental & Clinical Pharmacology. 2009; 23: 757–765. doi: 10.1111/j.1472-8206.2009.00721.x.
54.
Palabiyik S, Karakus E, Halici Z, et al. The protective effects of carvacrol and thymol against paracetamol-induced toxicity on human hepatocellular carcinoma cell lines (HepG2). Human & Experimental Toxicology. 2016; 35. doi: 10.1177/0960327115627688.
55.
Bakır M, Geyikoglu F, Colak S, et al. The carvacrol ameliorates acute pancreatitis-induced liver injury via antioxidant response. Cytotechnology. 2016; 68: 1131–1146. doi: 10.1007/s10616-015-9871-z.
56.
Abd El Aal HA, Ahmed LA, Hassan WA, et al. Combination of carvacrol with simvastatin improves the lipid-lowering efficacy and alleviates simvastatin side effects. Journal of Biochemical and Molecular Toxicology. 2017; 31: e21981. doi: 10.1002/jbt.21981.
57.
Jayakumar S, Madankumar A, Asokkumar S, et al. Potential preventive effect of carvacrol against diethylnitrosamine-induced hepatocellular carcinoma in rats. Mol Cell Biochem. 2012; 360: 51–60. doi: 10.1007/ s11010-011-1043-7.
58.
Rajan B, Ravikumar R, Premkumar T, et al. Carvacrol attenuates N-nitrosodiethylamine induced liver injury in experimental Wistar rats. Food Science and Human Wellness. 2015; 4: 66–74. doi: https:// doi.org/10.1016/j.fshw.2015.04.002.
59.
Khazdair MR, Alavinezhad A, Boskabady MH. Carvacrol ameliorates haematological parameters, oxidant/antioxidant biomarkers and pulmonary function tests in patients with sulphur mustard-induced lung disorders: A randomized double-blind clinical trial. J Clin Pharm Ther. 2018; 43: 664–674. doi: 10.1111/jcpt.12684.
60.
Khazdair MR, Boskabady MH. A double-blind, randomized, placebo-controlled clinical trial on the effect of carvacrol on serum cytokine levels and pulmonary function tests in sulfur mustard induced lung injury. Cytokine. 2019; 113: 311–318. doi: 10.1016/j.cyto.2018.07.031.
61.
Alam K, Nagi MN, Badary OA, et al. The protective action of thymol against carbon tetrachloride hepatotoxicity in mice. Pharmacological Research. 1999; 40: 159–163. doi: https://doi.org/10.1006/phrs.1....
62.
Özkan A, Erdogan A. A comparative evaluation of antioxidant and anticancer activity of essential oil from Origanum onites (Lamiaceae) and its two major phenolic components. Turkish Journal of Biology. 2011; 35: 735–742. doi: 10.3906/biy-1011-170.
63.
Al-Awaida W, Akash M. Protective role of aqueous medicinal herbal extracts against oxidative stress on Glucose-6-phosphate dehydrogenase activity and RBC fragility. Life Science Journal. 2014; 11: 385–391.
64.
Rašković A, Pavlović N, Kvrgić M, et al. Effects of pharmaceutical formulations containing thyme on carbon tetrachloride-induced liver injury in rats. BMC Complement Altern Med. 2015; 15: 442. doi: 10.1186/ s12906-015-0966-z.
65.
Placha I, Takacova J, Ryzner M, et al. Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. J British Poultry Science. 2014; 55: 105–114.
66.
Nobakht M, Darmanikuhi H, Mohiti-Asli M. Effects of Zataria multiflora boiss (thyme) extract and dietary fat on performance and carcass antioxidant properties in broiler chicks. Animal Science Journal. 2017; 117: 117–128.
67.
Rubió L, Serra A, Chen CY, et al. Effect of the co-occurring components from olive oil and thyme extracts on the antioxidant status and its bioavailability in an acute ingestion in rats. Food Funct. 2014; 5: 740–747. doi: 10.1039/c3fo60446b.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.