Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
A Phase I study evaluating the pharmacokinetic profile of a novel oral analgesic propoxazepam
1
A.V. Bogatsky Physico-Chemical Institute of the National Academy of Sciences, Odesa, Ukraine
Corresponding author
Vitalii Larionov
A.V. Bogatsky Physical-Chemical Institute of National Academy of Sciences of Ukraine, Lyustdorfskaya doroga, 86, 65080, Odesa, Ukraine
A.V. Bogatsky Physical-Chemical Institute of National Academy of Sciences of Ukraine, Lyustdorfskaya doroga, 86, 65080, Odesa, Ukraine
J Pre Clin Clin Res. 2023;17(3):138-144
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Propoxazepam, 7-bromo-5 - (o-chlorophenyl)-3-propoxy – 1,2-dihydro – 3H-1,4- benzodiazepin-2-one, in the models of nociceptive and neuropathic pain showed significant analgesic activity. The aim of the study was to investigate the pharmacokinetics of propoxazepam and its metabolites after a single oral dose in healthy volunteers.
Material and methods:
12 volunteers were orally dosed with 5 mg propoxazepam under fasting conditions. Blood samples were collected up to 72 hours after administration and propxazepam extracted with liquid-phase extraction and analyzed with high-performance liquid chromatography–tandem mass spectrometry.
Results:
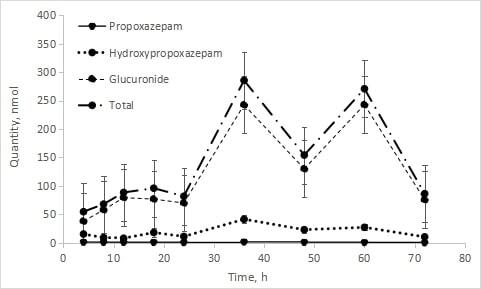
Maximum propoxazepam concentration (22.276 ng/mL) was reached in blood by 4 hours after administration. It had a large volume of distribution (~6.3 L/kg), the elimination half-life 30.11 hours, MRT 37.77 hours, common clearance – 9062.929 mL/hour. Both propoxazepam and its metabolites (3-hydroxy derivative and glucuronide) were detected in the urine of volunteers. The urinary excretion rate of propoxazepam is proportional to its concentration in plasma. Only a small amount of unchanged propoxazepam was excreted with urine – 0.062 % of the administered dose. Renal clearance – 6.46 mL/hour.
Conclusions:
A single dose (5 mg) of Propoxazepam administered orally showed good tolerability, pharmacokinetically characterized by rapid absorption, slow elimination and low quantities of unchanged parent urinary excreted. The oxidized metabolite (3-hydroxypropoxazepam) and its glucuronide were excreted with urin, a total of up to 10.5% of the administered dose, which indicates a high degree of metabolism and possible hepatointestinal circulation.
Propoxazepam, 7-bromo-5 - (o-chlorophenyl)-3-propoxy – 1,2-dihydro – 3H-1,4- benzodiazepin-2-one, in the models of nociceptive and neuropathic pain showed significant analgesic activity. The aim of the study was to investigate the pharmacokinetics of propoxazepam and its metabolites after a single oral dose in healthy volunteers.
Material and methods:
12 volunteers were orally dosed with 5 mg propoxazepam under fasting conditions. Blood samples were collected up to 72 hours after administration and propxazepam extracted with liquid-phase extraction and analyzed with high-performance liquid chromatography–tandem mass spectrometry.
Results:
Maximum propoxazepam concentration (22.276 ng/mL) was reached in blood by 4 hours after administration. It had a large volume of distribution (~6.3 L/kg), the elimination half-life 30.11 hours, MRT 37.77 hours, common clearance – 9062.929 mL/hour. Both propoxazepam and its metabolites (3-hydroxy derivative and glucuronide) were detected in the urine of volunteers. The urinary excretion rate of propoxazepam is proportional to its concentration in plasma. Only a small amount of unchanged propoxazepam was excreted with urine – 0.062 % of the administered dose. Renal clearance – 6.46 mL/hour.
Conclusions:
A single dose (5 mg) of Propoxazepam administered orally showed good tolerability, pharmacokinetically characterized by rapid absorption, slow elimination and low quantities of unchanged parent urinary excreted. The oxidized metabolite (3-hydroxypropoxazepam) and its glucuronide were excreted with urin, a total of up to 10.5% of the administered dose, which indicates a high degree of metabolism and possible hepatointestinal circulation.
ACKNOWLEDGEMENTS
The authors exprss their gratitude to SLC ‘INTERCHEM’ for providing funds and a material/instrumental base, and to Heorhii Mal`tsev for technical and methodological consultations.
Golovenko M, Reder A, Zupanets I, Bezugla N, Larionov V, Valivodz I. A Phase I study evaluating the pharmacokinetic profile of a novel oral analgesic propoxazepam. J Pre-Clin Clin Res. 2023; 17(3): 138–144. doi: 10.26444/jpccr/169426
REFERENCES (20)
1.
Katz J, Rosenbloom BN. The golden anniversary of Melzack and Wall’s gate control theory of pain: Celebrating 50 years of pain research and management. Pain Res Manag. 2015;20(6):285–6. doi: 10.1155/2015/865487.
2.
Li C, Lei Y, Tian Y, Xu S, Shen X, Wu H, Bao S, Wang F. The etiological contribution of GABAergic plasticity to the pathogenesis of neuropathic pain. Mol Pain. 2019;15:1744806919847366. doi: 10.1177/1744806919847366.
3.
Huang YJ, Lee KH, Murphy L, Garraway SM, Grau JW. Acute spinal cord injury (SCI) transforms how GABA affects nociceptive sensitization. Exp Neurol. 2016;285(Pt A):82–95. doi: 10.1016/j.expneurol.2016.09.005.
4.
Castellano D, Shepard RD, Lu W. Looking for Novelty in an “Old” Receptor: Recent Advances Toward Our Understanding of GABAARs and Their Implications in Receptor Pharmacology. Front Neurosci. 2021;14:616298. doi: 10.3389/fnins.2020.616298.
5.
Zeilhofer HU, Neumann E, Munro G. Spinal GABAA receptors for pain control: back to the future? Br J Anaesth. 2019;123(2):e176-e179. doi: 10.1016/j.bja.2019.01.030.
6.
Engin E. GABAA receptor subtypes and benzodiazepine use, misuse, and abuse. Front Psychiatry. 2023;13:1060949. doi:10.3389/fpsyt.2022.1060949.
7.
Kowalczyk P, Kulig K. GABA system as a target for new drugs. Curr Med Chem. 2014;21(28):3294–309. doi:10.2174/0929867321666140601202158.
8.
Bhagat K, Singh JV, Pagare PP, Kumar N, Sharma A, Kaur G, Kinarivala N, Gandu S, Singh H, Sharma S, Bedi PMS. Rational approaches for the design of various GABA modulators and their clinical progression. Mol Divers. 2021;25(1):551–601. doi:10.1007/s11030-020-10068-4.
9.
Chincholkar M. Gabapentinoids: pharmacokinetics, pharmacodynamics and considerations for clinical practice. Br J Pain. 2020;14(2):104–114. doi:10.1177/2049463720912496.
10.
Golovenko NYa, Larionov VB, Reder AS, Valivodz IP. An effector analysis of the interaction of propoxazepam with antagonists of GABA and glycine receptors. Neurochemical Journal. 2017;11(4):302–308.
11.
Golovenko M, Reder A, Andronati S, Larionov V. Evidence for the involvement of the GABA-ergic pathway in the anticonvulsant and antinociception activity of Propoxazepam in mice and rats. J Pre-Clin Clin Res. 2019;13(3):99–105.
12.
Golovenko NYa, Larionov VB, Andronati SA, Valivodz IP, Yurpalova TA. Pharmacodynamics of interaction between Propoxazepam and a GABA-benzodiazepine receptor-ionophor complex. Neurophysiology. 2018;50(1):2–10.
13.
Golovenko NYa, Voloshchuk NI, Andronati SA, et al. Antinociception induced by a novel benzodiazepine receptor agonist and bradykinin receptor antagonist in rodent acute and chronic pain models. EJBPS. 2018;5(12):79–88.
14.
Guideline on strategies to identify and mitigate risks for first-in-human clinical trials with Investigational Medicinal Products. EMEA/CHMP/SWP/28367/07.
15.
Guidance for Industry Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. FDA/CDER, July 2005.
16.
Directive 2001/20/EC of the European Parliament and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official Journal of the European Communities L 121, 1.5.2001.
17.
Guidance for organizations performing in vivo bioequivalence studies / WHO Expert Committee on Specifications for Pharmaceutical Preparations Fiftieth report, Annex 9 (2016).
18.
Golovenko M, Reder A, Larionov V, Andronati S. Metabolic profile and mechanisms reaction of receptor GABA-targeted propoxazepam in human hepatocytes. Biotechnologia Acta. 2022;15(1):5–12.
19.
Mizuno K, Katoh M, Okumura H, et al. Metabolic activation of benzodiazepines by CYP3A4. Drug Metab Dispos. 2009;37(2):345–51. doi:10.1124/dmd.108.024521.
20.
Seo KA, Kim HJ, Jeong ES, et al. In vitro assay of six UDP-glucuronosyltransferase isoforms in human liver microsomes, using cocktails of probe substrates and liquid chromatography-tandem mass spectrometry. Drug Metab Dispos. 2014;42(11):1803–10. doi:10.1124/dmd.114.058818.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.