Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
CASE REPORT
A 9-year-old patient with severe haemophilia A complicated by factor VIII inhibitor treated with emicizumab – case report and literature review
1
Medical University, Lublin, Poland
2
Student Research Group at the Department of Hematology, Oncology and Children’s Transplantology, Medical
University, Lublin, Poland
3
Department of Transfusion Medicine, Children`s University Hospital, Lublin, Poland
Corresponding author
Justyna Małgorzata Tomasik
Medical University of Lublin, Aleje Racławickie 1, 20-059, Lublin, Poland
Medical University of Lublin, Aleje Racławickie 1, 20-059, Lublin, Poland
J Pre Clin Clin Res. 2024;18(2):128-134
KEYWORDS
TOPICS
ABSTRACT
Introduction:
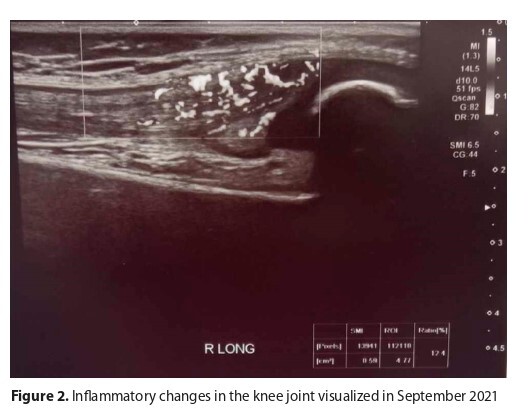
The case is presented of a 10-year-old boy with severe Haemophilia A, in whom standard therapy led to the development of an inhibitor to factor VIII. Immunological tolerance induction (ITI) did not yield the expected result, and inhibitor titers were not reduced. Due to numerous recurrent bleeds, prophylaxis with activated prothrombin complex concentrate (aPCC) was initiated, with recombinant factor VIIa administered in the case of bleeding episodes. Despite second and third-line ITI treatments, desired outcomes were not achieved, leading to therapy discontinuation and initiation of emicizumab treatment. The new therapy significantly reduced bleeding occurrences.
Results:
This case clearly illustrates the greater efficacy of emicizumab compared to other drugs, confirming its application in patients with developed factor VIII inhibitors where ITI therapy was ineffective. Prophylaxis with this antibody significantly reduces bleeding frequency and improves the patient’s quality of life.
The case is presented of a 10-year-old boy with severe Haemophilia A, in whom standard therapy led to the development of an inhibitor to factor VIII. Immunological tolerance induction (ITI) did not yield the expected result, and inhibitor titers were not reduced. Due to numerous recurrent bleeds, prophylaxis with activated prothrombin complex concentrate (aPCC) was initiated, with recombinant factor VIIa administered in the case of bleeding episodes. Despite second and third-line ITI treatments, desired outcomes were not achieved, leading to therapy discontinuation and initiation of emicizumab treatment. The new therapy significantly reduced bleeding occurrences.
Results:
This case clearly illustrates the greater efficacy of emicizumab compared to other drugs, confirming its application in patients with developed factor VIII inhibitors where ITI therapy was ineffective. Prophylaxis with this antibody significantly reduces bleeding frequency and improves the patient’s quality of life.
Tomasik JM, Szuba J, Chrostowski K, Woźnica-Karczmarz I. A 9-year-old patient with severe haemophilia A complicated by factor VIII inhibitor
treated with emicizumab – case report and literature review. J Pre-Clin Clin Res. 2024; 18(2): 128–134. doi: 10.26444/jpccr/187549
REFERENCES (33)
1.
Windyga J, Chojnowski K, Klukowska A, et al. Part I: Guidelines for the management of uncomplicated haemophilia A and B with inhibitors to factor VIII and IX (updated edition). Acta Haematol Pol. 2016;47(2):86– 114. doi:10.1016/J.ACHAEM.2016.04.009.
2.
Windyga J, Chojnowski K, Klukowska A, et al. Part II: Guidelines for the management of complicated haemophilia A and B with inhibitors to factor VIII and IX (2nd edition). Acta Haematol Pol. 2017;48(3):137–159. doi:10.1016/J.ACHAEM.2017.08.001.
3.
Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Guidelines for the management of haemophilia. Haemophilia. 2013;19(1):e1–e47. doi:10.1111/J.1365-2516.2012.02909.X.
4.
Makris M, Kasper C. The World Federation of Haemophilia guideline on management of haemophilia. Haemophilia. 2013;19(1):1–1. doi:10.1111/HAE.12074.
5.
Garcia J, Hammer MR, Zia A. Serious bleeds in pediatric persons with haemophilia A on emicizumab prophylaxis. Res Pract Thromb Haemost. 2023;7(8):102238. doi:10.1016/J.RPTH.2023.102238.
6.
Levy-Mendelovich S, Atia N, Budnik I, et al. Factor VIII inhibitors in haemophilia A treated with emicizumab: longitudinal follow up of outcomes. Res Pract Thromb Haemost. 2023;7(4). doi:10.1016/J. RPTH.2023.100278.
7.
Lillicrap D, Fijnvandraat K, Young G, Mancuso ME. Patients with haemophilia A and inhibitors: prevention and evolving treatment paradigms. Expert Rev Hematol. 2020;13(4):313–321. doi:10.1080/17 474086.2020.1739518.
8.
Callaghan MU, Negrier C, Paz-Priel I, et al. Long-term outcomes with emicizumab prophylaxis for haemophilia A with or without FVIII inhibitors from the HAVEN 1–4 studies. Blood. 2021;137(16):2231. doi:10.1182/BLOOD.2020009217.
9.
Mahlangu JN. Bispecific Antibody Emicizumab for Haemophilia A: A Breakthrough for Patients with Inhibitors. BioDrugs. 2018;32(6):561–570. doi:10.1007/S40259-018-0315-0/METRICS.
10.
Yoneyama K, Schmitt C, Portron A, et al. Clinical pharmacology of emicizumab for the treatment of haemophilia A. Expert Rev Clin Pharmacol. 2023;16(9):775–790. doi:10.1080/17512433.2023.2243213.
11.
Franchini M, Mannucci PM. Past, present and future of haemophilia: a narrative review. Orphanet J Rare Dis. 2012;7(1):24. doi:10.1186/1750-1172-7-24.
12.
Oudat R, Al-Maharmeh M, Al-Ghrayeb R, Ogeilat T, Mustafa MK. Prevalence of FVIII Inhibitors Among Children with Haemophilia A:Experience at the Jordanian Royal Medical Services. Medical Archives. 2020;74(3):187. doi:10.5455/MEDARH.2020.74.187-190.
13.
Odnoczko E, Windyga J. Genetic testing in the diagnosis of haemophilia A. Published online 2014.
14.
Pediatrics after Graduation – Haemophilia in children. Accessed March 11, 2024.https://podyplomie.pl/pediatri... 12198,hemofilia-u-dzieci.
15.
Berntorp E, Fischer K, Hart DP, et al. Haemophilia. Nature Reviews Disease Primers 2021;7(1):1–19. doi:10.1038/s41572-021-00278-x.
16.
Peyvandi F, Garagiola I, Young G. The past and future of haemophilia: diagnosis, treatments, and its complications. The Lancet. 2016;388(10040):187–197. doi:10.1016/S0140-6736(15)01123-X.
17.
Nugent D, O’Mahony B, Dolan G. Value of prophylaxis vs ondemand treatment: Application of a value framework in haemophilia. Haemophilia. 2018;24(5):755–765. doi:10.1111/HAE.13589.
18.
Haemophilia in children yesterday and today | Health Service. Accessed March 11, 2024. https://zdrowie.pap.pl/wywiady....
19.
Haemophilia in questions and answers. Guide for patients and their families. | Pediatric Oncology.pl. Accessed March 11, 2024. https://onkologia-dziecieca.pl....
20.
Samelson-Jones BJ, George LA. Haemophilia care: the only constant is change. Br J Haematol. 2021;194(5):805–807. doi:10.1111 BJH.17661.
21.
Letowska MK, Zawilska K, Klukowska A, et al. Immune Tolerance Induction (ITI) in Paediatric and Adult Patients with Haemophilia a (HA) and High-Titre (HT) Factor VIII (FVIII) Inhibitor – Real Life Data. Blood. 2019;134(Supplement_1):4942. doi:10.1182/BLOOD-2019-122422.
22.
Fassel H, McGuinn C. Haemophilia: factoring in new therapies. Br J Haematol. 2021;194(5):835–850. doi:10.1111/BJH.17580.
23.
Le Quellec S. Clinical Evidence and Safety Profile of Emicizumab for the Management of Children with Haemophilia A. Drug Des Devel Ther. 2020;14:469. doi:10.2147/DDDT.S167731.
24.
Windyga J, Chojnowski K, Klukowska A, et al. Emicizumab (Hemlibra®) in haemophilia A patients with inhibitors against factor VIII — guidelines of the Group for Haemostasis of the Polish Society of Haematology and Transfusion Medicine. J Transf Med. 2020;13(3):165–75. doi:10.5603/ JTM.2020.0006.
26.
Hemlibra® (emicizumab) summary of product characteristics. EMA. https://www.ema.europa.eu/en/d... (accessed: 2024.11.03).
27.
Young G, Liesner R, Chang T, et al. A multicenter, open-label phase 3 study of emicizumab prophylaxis in children with haemophilia A with inhibitors. Blood. 2019;134(24):2127–2138. doi:10.1182/ BLOOD.2019001869.
28.
Pedro Alcedo Andrade by E, Mannuccio Mannucci P, Kessler CM, et al. Emicizumab: the haemophilia A game changer.Haematologica. Published online November 2, 2020:0–0. doi:10.3324/HAEMATOL.2022.282099.
29.
Muniz RL, Camelo RM, Araújo MS, et al. Efficacy/effectiveness and safety of emicizumab prophylaxis of people with haemophilia A: a systematic review and meta-analysis. Expert Rev Hematol. 2023;16(12):1087–1097. doi:10.1080/17474086.2023.2293096.
30.
Glonnegger H, Andresen F, Kapp F, Malvestiti S, Büchsel M, Zieger B. Emicizumab in children: bleeding episodes and outcome before and after transition to Emicizumab. BMC Pediatr. 2022;22(1). doi:10.1186/S12887-022-03546-1.
31.
Liu G, Huang K, Li G, et al. Real-world experience of emicizumab prophylaxis in young children with haemophilia A: retrospective data from China. Front Pediatr. 2022;10. doi:10.3389/FPED.2022.992267.
32.
Hassan E, Jonathan L, Jayashree M. Real-world experience on the tolerability and safety of emicizumab prophylaxis in paediatric patients with severe haemophilia A with and without FVIII inhibitors. Haemophilia. 2021;27(6):e698-e703. doi:10.1111/HAE.14432.
33.
Ahn JH, Jung N, Shim YJ, Kim HS. Emicizumab prophylaxis in a Korean child with severe haemophilia A and high titer inhibitor: a case report. Blood Res. 2021;56(1):44. doi:10.5045/BR.2021.2020184.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.