REVIEW PAPER
Botulinum in selected neurological disorders - review
1
Medical University, Lublin, Poland
2
Department of Neurology, Medical University, Poland
Corresponding author
J Pre Clin Clin Res. 2021;15(4):176-183
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Botulinum toxin (BoNT) has been used in medicine for many years. BoNT, prevents acetylcholine from being released into synapses, causing flaccid muscles paralysis. The article reviews the current knowledge of botulinum toxin application in the treatment of neurological diseases, focusing on therapeutic efficacy and side-effects. The aim of the review is to analyze the largest possible number of neurological conditions in which the application of botulinum toxin was considered.
Materials and method:
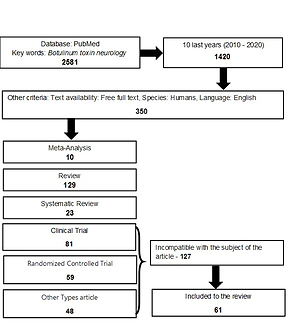
A literature review of the last 10 years was conducted using the key words: ‘botulinum toxin neurology’ in the PubMed database, with the search criteria: clinical trials, free full text in English,research on humans. 2,581 manuscripts were found. After initial analysis, 188 publications were selected for further elaboration. Finally, 61 compatible publications were identified and included in the review.
Abbreviated description of the state of knowledge:
In the treatment of Tourette’s syndrome with BoNT, despite the reduction in tics frequency, patients did not report any benefits. There are promising reports on the reduction of the intensity of neuropathic pain and neuralgia in trigeminal neuralgia after the use of BoNT-A. Improvement in rest tremor has been seen in patients with Parkinson’s disease. Administration of BoNT-A among patients with bruxism was associated with improved sleep quality and reduction of the symptoms intensity. Research confirms the effectiveness of using BoNT in the treatment of bothersome symptoms occurring in migraine, drooling, cervical dystonia and neurogenic bladder.
Conclusions:
Although the use of BoNT in neurological diseases is widely studied and used, the appropriate administration technique and safety of use are worth further research.
Botulinum toxin (BoNT) has been used in medicine for many years. BoNT, prevents acetylcholine from being released into synapses, causing flaccid muscles paralysis. The article reviews the current knowledge of botulinum toxin application in the treatment of neurological diseases, focusing on therapeutic efficacy and side-effects. The aim of the review is to analyze the largest possible number of neurological conditions in which the application of botulinum toxin was considered.
Materials and method:
A literature review of the last 10 years was conducted using the key words: ‘botulinum toxin neurology’ in the PubMed database, with the search criteria: clinical trials, free full text in English,research on humans. 2,581 manuscripts were found. After initial analysis, 188 publications were selected for further elaboration. Finally, 61 compatible publications were identified and included in the review.
Abbreviated description of the state of knowledge:
In the treatment of Tourette’s syndrome with BoNT, despite the reduction in tics frequency, patients did not report any benefits. There are promising reports on the reduction of the intensity of neuropathic pain and neuralgia in trigeminal neuralgia after the use of BoNT-A. Improvement in rest tremor has been seen in patients with Parkinson’s disease. Administration of BoNT-A among patients with bruxism was associated with improved sleep quality and reduction of the symptoms intensity. Research confirms the effectiveness of using BoNT in the treatment of bothersome symptoms occurring in migraine, drooling, cervical dystonia and neurogenic bladder.
Conclusions:
Although the use of BoNT in neurological diseases is widely studied and used, the appropriate administration technique and safety of use are worth further research.
Rocka A, Piędel F, Jasielski PP, Piwek M, Petit V, Rejdak K. Botulinum in selected neurological disorders – review. J Pre-Clin Clin Res. 2021;
15(4): 176–183. doi: 10.26444/jpccr/142880
REFERENCES (82)
1.
Jabbari B. Botulinum Toxin Treatment in Neurology. Semin Neurol. 2016; 36(1): 3–4. https://doi.org/10.1055/s-0036....
2.
Dressler D. Therapeutically relevant features of botulinum toxin drugs.Toxicon. 2020; 175: 64–68. https://doi.org/10.1016/j.toxi....
3.
Pirazzini M, Rossetto O, Eleopra R, et al. Botulinum Neurotoxins: Biology, Pharmacology, and Toxicology. Pharmacol Rev. 2017; 69(2): 200–235. https://dx.doi.org/10.1124%2Fp....
4.
Tater P, Pandey S. Botulinum toxin in movement disorders. Neurol India. 2018; 66: 79–89. https://doi.org/10.4103/0028–3....
5.
Carruthers A. History of the clinical use of botulinum toxin A and B. Clin Dermatol. 2003; 21: 469–472. https://doi.org/10.1016/j.clin....
6.
López del Val LJ, Castro García A. Toxina Botulínica: Aplicaciones Terapéuticas. 2nd ed. Barcelona, Spain: Masson; 2002. p. 3–21.
7.
Zhang S, Masuyer G, Zhang J, et al. Identification and characterization of a novel botulinum neurotoxin. Nat Commun. 2017; 8: 14130. https://doi.org/10.1038/ncomms....
8.
Jahn R, Scheller R.H. SNAREs-engines for membrane fusion. Nat Rev Mol Cell Biol. 2006; 7: 631–643. https://doi.org/10.1038/nrm200....
9.
Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009; 323: 474–477. https://doi.org/10.1126/scienc....
10.
Turna IF, Erhan B, Gunduz NB, et al. The effects of different injection techniques of botulinum toxin in post-stroke patients with plantar flexor spasticity. Acta Neurol Belg. 2020; 120(3): 639–643. https://doi.org/10.1007/s13760....
11.
Santamato A, Micello MF, Panza F, et al. Can botulinum toxin type A injection technique influence the clinical outcome of patients with post-stroke upper limb spasticity? A randomized controlled trial comparing manual needle placement and ultrasound-guided injection techniques. J Neurol Sci. 2014; 347(1–2): 39–43. https://doi.org/10.1016/j.jns.....
12.
Porta M, Gamba M, Bertacchi G, et al. Treatment of sialorrhoea with ultrasound guided botulinum toxin type injection in patients with neurological disorders. J Neurol Neurosurg Psychiatry. 2001; 70: 538–540. https://dx.doi.or/10.1136%2Fjn....
13.
Tilton A, Russman B, Aydin R, et al. AbobotulinumtoxinA (Dysport®) Improves Function According to Goal Attainment in Children With Dynamic Equinus Due to Cerebral Palsy. J Child Neurol. 2017; 32(5): 482–487. https://doi.org/10.1177/088307....
14.
Bachoud-Lévi AC, Ferreira J, Massart R, et al. International Guidelines for the Treatment of Huntington’s Disease. Front Neurol. 2019; 3: 10: 710. https://doi.org/10.3389/fneur.....
15.
Richardson D, Sheean G, Werring D, et al. Evaluating the role of botulinum toxin in the management of focal hypertonia in adults. J Neurol Neurosurg Psychiatry 2000; 69: 499–506. https://doi.org/10.1136/jnnp.6....
16.
Sławek J, Bogucki A, Budrewicz S, et al. Leczenie toksyną botulinową spastyczności kończyny dolnej po udarze mózgu — rekomendacje Sekcji Schorzeń Pozapiramidowych Polskiego Towarzystwa Neurologicznego, Polskiego Towarzystwa Choroby Parkinsona i Innych Zaburzeń Ruchowych oraz Interdyscyplinarnej Grupy Ekspertów. Pol Przegl Neurol. 2016; 12(2): 65–79. https://journals.viamedica.pl/....
17.
Watkins CL, Leathley MJ, Gregson JM, et al. Prevalence of spasticity post stroke. Clin Rehabil. 2002; 16: 515–522. https://doi.org/10.1191/026921....
18.
Sławek J, Koziorowski D, Dec-Ćwiek M, et al. Rekomendacje interdyscyplinarnej grupy ekspertów w zakresie kompleksowego i długofalowego leczenia spastyczności toksyną botulinową typu A. Pol Przegl Neurol. 2018; 14(2): 47–59. https://journals.viamedica.pl/....
19.
Sławek J. Toksyna botulinowa w leczeniu spastyczności kończyny górnej. Pol Przegl Neurol. 2015; 11(4): 190–201. https://journals.viamedica.pl/....
20.
Gordon MF, Brashear A, Elovic E, et al. BOTOX Poststroke Spasticity Study Group. Repeated dosing of botulinum toxin type A for upper limb spasticity following stroke. Neurology. 2004; 63(10): 1971–1973. https://doi.org/10.1212/01.wnl....
21.
Lagalla G, Danni M, Reiter F, et al. Post-stroke spasticity management with repeated botulinum toxin injections in the upper limb. Am J Phys Med Rehab. 2000; 79(4): 377–384. https://doi.org/10.1097/000020....
22.
Baricich A, Grana E, Carda S, et al. High doses of onabotulinumtoxinA in post-stroke spasticity: a retrospective analysis. J Neural Transm (Vienna). 2015; 122(9): 1283–1287. https://doi.org/10.1007/s00702....
23.
Picelli A, Baricich A, Cisari C, et al. The Italian real-life post-stroke spasticity survey: unmet needs in the management of spasticity with botulinum toxin type A. Funct Neurol. 2017; 32(2): 89–96. https://doi.org/10.11138/fneur....
24.
BOTOX (onabotulinumtoxinA) for injection, for intramuscular, intradetrusor, or intradermal use Initial U.S. Approval: 1989. https://www.accessdata.fda.gov....
25.
Van Der Walt A, Sung S, Spelman T, et al. A double-blind, randomized, controlled study of botulinum toxin type A in MS-related tremor. Neurology. 2012; 79(1): 92–99. https://doi.org/10.1212/wnl.0b....
26.
Restivo DA, Marchese-Ragona R, Patti F, et al. Botulinum toxin improves dysphagia associated with multiple sclerosis. Eur J Neurol. 2011; 18(3): 486–490. https://doi.org/10.1111/j.1468....
27.
Capriotti T, Terzakis K. Parkinson Disease. Home Healthc Now. 2016; 34(6): 300–307. https://doi.org/10.1097/nhh.00....
28.
Rieu I, Degos B, Castelnovo G, et al. Incobotulinum toxin A in Parkinson’s disease with foot dystonia: A double blind randomized trial. Parkinsonism Relat Disord. 2018; 46: 9–15. https://doi.org/10.1016/j.park....
29.
Bruno V, Freitas ME, Mancini D. Botulinum Toxin Type A for Pain in Advanced Parkinson’s Disease. Can J Neurol Sci. 2018; 45(1): 23–29. https://doi.org/10.1017/cjn.20....
30.
Mittal S, Machado D, Richardson D, et al. Botulinum Toxin in Parkinson Disease Tremor: A Randomized, Double-Blind, Placebo-Controlled Study With a Customized Injection Approach. Mayo Clin Proc. 2017; 92(9): 1359–1367. https://doi.org/10.1016/j.mayo....
31.
Jinnah HA, Factor SA. Diagnosis and treatment of dystonia. Neurol Clin. 2015; 33: 77–100. https://doi.org/10.1016/j.ncl.....
33.
Pirazzini M, Rossetto O, Eleopra R, et al. Botulinum Neurotoksyny: biologia, farmakologia i toksykologia. Pharmacol Rev. 2017; 69: 200–235. https://doi.org/10.1124/pr.116....
34.
Huang L, Chen HX, Ding XD, et al. Efficacy analysis of ultrasound-guided local injection of botulinum toxin type A treatment with orthopedic joint brace in patients with cervical dystonia. Eur Rev Med Pharmacol Sci. 2015; 19(11): 1989–1993. https://pubmed.ncbi.nlm.nih.go....
35.
Hu W, Rundle-Gonzalez V, Kulkarni SJ, et al. A randomized study of botulinum toxin versus botulinum toxin plus physical therapy for treatment of cervical dystonia. Parkinsonism Relat Disord. 2019; 63: 195–198. https://doi.org/10.1016/j.park....
36.
Samotus O, Lee J, Jog M. Personalized botulinum toxin type A therapy for cervical dystonia based on kinematic guidance. J Neurol. 2018; 265(6): 1269–1278. https://doi.org/10.1007/s00415....
37.
Jinnah HA, Comella CL, Perlmutter J, et al. Dystonia Coalition Investigators. Longitudinal studies of botulinum toxin in cervical dystonia: Why do patients discontinue therapy?. Toxicon. 2018; 147: 89–95. https://doi.org/10.1016/j.toxi....
38.
Ansved T, Odergren T, Borg K. Muscle fiber atrophy in leg muscles after botulinum toxin type A treatment of cervical dystonia. Neurology. 1997; 48: 1440–1442. https://doi.org/10.1212/WNL.48....
39.
Núñez Medrano JA, Fernández E, Georgescu D, et al. Consensus of the Iberoamerican Oculoplastic Society for diagnosis and management of facial dystonia. Arch Soc Esp Oftalmol. 2019; 94(9): 436–440. https://doi.org/10.1016/j.ofta....
40.
Thenganatt MA, Jankovica J. Recent Advances in Understanding and Managing Tourette Syndrome; Version 1. F1000Res. 2016; 5: F1000 Faculty Rev-152. https://dx.doi.org/10.12688%2F....
41.
Pandey S, Srivanitchapoom P, Kirubakaran R, et al. Botulinum toxin for motor and phonic tics in Tourette’s syndrome; Cochrane Database Syst Rev. 2018; 1(1): CD012285. https://doi.org/10.1002/146518....
42.
Roth J. The colorful spectrum of Tourette syndrome and its medical, surgical and behavioral therapies; Parkinsonism Relat Disord. 2018; 46 Suppl 1: 75–79. https://doi.org/10.1016/j.park....
43.
Marras C, Andrews D, Sime E, et al. Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology. 2001; 56(5): 605–610. https://doi.org/10.1212/wnl.56....
44.
Kim J, Kim JY, Lee JM, et al. Progressive Cervical Spondylotic Myelopathy Caused by Tic Disorders in a Young Adult with Tourette Syndrome. Korean J Neurotrauma. 2019; 15(2): 199–203. https://doi.org/10.13004/kjnt.....
45.
Louis ED, Marder K, Cote L, et al. Differences in the prevalence of essential tremor among elderly African, American, whites and Hispanics in Northern Manhattan, NY. Arch Neurol. 1995; 52: 1201–1205. https://doi.org/10.1001/archne....
46.
Zesiewicz TA, Kuo SH. Essential tremor. BMJ Clin Evid. 2015; 15: 1206. https://www.ncbi.nlm.nih.gov/p....
47.
Brin MF, Lyons KE, Doucette J, et al. A randomized, double masked, controlled trial of botulinum toxin type A in essential hand tremor. Neurology. 2001; 56: 1523–1528. https://doi.org/10.1212/wnl.56....
48.
Ondo WG, Simmons JH, Shahid MH, et al. Onabotulinum toxin-A injections for sleep bruxism: A double-blind, placebo-controlled study. Neurology. 2018; 90(7): 559–564. https://doi.org/10.1212/wnl.00....
49.
Shim YJ, Lee HJ, Park KJ, et al. Botulinum Toxin Therapy for Managing Sleep Bruxism: A Randomized and Placebo-Controlled Trial. Toxins (Basel). 2020; 12(3): 168. https://doi.org/10.3390/toxins....
50.
Pardo NB, Kerstein RB, Júnior MC, et al. Botulinum toxin type A for controlling bruxism assessed with computerized occlusal analysis: A pilot study. Cranio. 2020; 2: 1–10. https://doi.org/10.1080/088696....
51.
Jadhao VA, Lokhande N, Habbu SG, et al. Efficacy of botulinum toxin in treating myofascial pain and occlusal force characteristics of masticatory muscles in bruxism. Indian J Dent Res. 2017; 28: 493–497. https://doi.org/10.4103/ijdr.i....
52.
Simpson D. Clinical trials of botulinum toxin in the treatment of spasticity. Muscle Nerve Suppl. 1997; 6: 169–175. https://doi.org/10.1002/(SICI)...+<169::AID-MUS11>3.0.CO;2-1.
53.
Amundsen CL, Komesu YM, Chermansky C, et al. Pelvic Floor Disorders Network. Two-Year Outcomes of Sacral Neuromodulation Versus OnabotulinumtoxinA for Refractory Urgency Urinary Incontinence: A Randomized Trial. Eur Urol. 2018; 74(1): 66–73. https://doi.org/10.1016/j.euru....
54.
Reitz A, Stöhrer M, Kramer G, et al. European experience of 200 cases treated with botulinum-A toxin injections into the detrusor muscle for urinary incontinence due to neurogenic detrusor overactivity. Eur Urol. 2004; 45(4): 510–515. https://doi.org/10.1016/j.euru....
55.
Banerjee KJ, Glasson C, O’Flaherty SJ. Parotid and submandibular botulinum toxin injections for sialorrhoea in children with cerebral palsy. Dev Med Child Neurol. 2006; 48: 883–887. https://doi.org/10.1017/s00121....
56.
Lovato A, Restivo DA, Ottaviano G, et al. Botulinum toxin therapy: Functional silencing of salivary disorders. Acta Otorhinolaryngol Ital. 2017; 37: 168–171. https://dx.doi.org/10.14639%2F....
57.
McGeachan AJ, Mcdermott CJ. Management of oral secretions in neurological disease. Pract Neurol. 2017; 17: 96–103. https://doi.org/10.1136/practn....
58.
Mazlan M, Rajasegaran S, Engkasan JP, et al. A Double-Blind Randomized Controlled Trial Investigating the Most Efficacious Dose of Botulinum Toxin-A for Sialorrhea Treatment in Asian Adults with Neurological Diseases. Toxins (Basel). 2015; 7(9): 3758–3770. https://doi.org/10.3390/toxins....
59.
Bekkers S, Delsing CP, Kok SE, et al. Randomized controlled trial comparing botulinum vs surgery for drooling in neurodisabilities. Neurolog y. 2019; 92(11): 1195–1204. https://doi.org/10.1212/WNL.00....
60.
Jost WH, Friedman A, Michel O, et al. SIAXI: Placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea. Neurology. 2019; 92(17): 1982–1991. https://doi.org/10.1212/wnl.00....
61.
Winner PK, Blumenfeld AM, Eross EJ, et al. Long-Term Safety and Tolerability of OnabotulinumtoxinA Treatment in Patients with Chronic Migraine: Results of the COMPEL Study. Drug Saf. 2019; 42(8): 1013–1024. https://doi.org/10.1007/s40264....
62.
Boczarska-Jedynak M, Sławek J. Praktyczne aspekty leczenia migreny przewlekłej toksyną botulinową typu A. Pol Przegl Neurol. 2017; 13(4): 189–198. https://journals.viamedica.pl/....
63.
Liberini P, Pari E, Gazzina S, et al. Technique of injection of onabotulinumtoxin A for chronic migraine: the PREEMPT injection paradigm. Neurol Sci. 2014; 35 Suppl 1: 41–43. https://doi.org/10.1007/s10072....
64.
Diener HC, Dodick DW, Aurora SK, et al. OnabotulinumtoxinA for treatment of chronic migraine: results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia. 2010; 30(7): 804–814. https://doi.org/10.1177/033310....
65.
Attal N, de Andrade DC, Adam F, et al. Safety and efficacy of repeated injections of botulinum toxin A in peripheral neuropathic pain (BOTNEP): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2016; 15(6): 555–565. https://doi.org/10.1016/s1474-....
66.
Xia JH, He CH, Zhang HF, et al. Botulinum toxin A in the treatment of trigeminal neuralgia. Int J Neurosci. 2016; 126(4): 348–353. https://doi.org/10.3109/002074....
67.
Shehata HS, El-Tamawy MS, Shalaby NM, et al. Botulinum toxin-type A: could it be an effective treatment option in intractable trigeminal neuralgia? J Headache Pain. 2013; 14(1): 92. https://doi.org/10.1186/1129-2....
68.
Wu KP, Chen CK, Lin SC, et al. Botulinum Toxin type A injections for patients with painful hallux valgus: a double-blind, randomized controlled study. Clin Neurol Neurosurg. 2015; 129 Suppl 1: 58–62. https://doi.org/10.1016/s0303-....
69.
Han ZA, Song DH, Oh HM, et al. Botulinum toxin type A for neuropathic pain in patients with spinal cord injury. Ann Neurol. 2016; 79(4): 569–578. https://doi.org/10.1002/ana.24....
70.
Santiago-Rosado LM, Lewison CS. Trismus. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. https://pubmed.ncbi.nlm.nih.go....
71.
Chang WD, Lee CL, Lin HY, et al. A Meta-analysis of Clinical Effects of Low-level Laser Therapy on Temporomandibular Joint Pain. J Phys Ther Sci. 2014; 26(8): 1297–1300. https://dx.doi.org/10.1589%2Fj....
72.
De Carli BM, Magro AK, Souza-Silva BN, et al. The effect of laser and botulinum toxin in the treatment of myofascial pain and mouth opening: A randomized clinical trial. J Photochem Photobiol B. 2016; 159: 120–123. https://doi.org/10.1016/j.jpho....
73.
Fietzek UM, Kossmehl P, Barthels A, et al. Botulinum toxin B increases mouth opening in patients with spastic trismus. Eur J Neurol. 2009; 16(12): 1299–1304. https://doi.org/10.1111/j.1468....
74.
Bradley EA, Hodge DO, Bartley GB. Benign essential blepharospasm among residents of Olmsted County, Minnesota, 1976 to 1995: an epidemiologic study. Ophthal Plast Reconstr Surg. 2003; 19: 177–181.https://doi.org/10.1097/01.iop....
75.
Hamill EB, Yen MT. The History of Blepharospasm in Medicine. Int Ophthalmol Clin. 2018; 58(1): 3–10. https://doi.org/10.1097/iio.00....
76.
Simpson DM, Hallett M, Ashman EJ, et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016; 86(19): 1818–1826. https://doi.org/10.1212/wnl.00....
77.
Yen MT. Developments in the treatment of benign essential blepharospasm. Curr Opin Ophthalmol. 2018; 29(5): 440–444. https://doi.org/10.1097/icu.00....
78.
Lee S, Park S, Lew H. Long-term Efficacy of Botulinum Neurotoxin-A Treatment for Essential Blepharospasm. Korean Ophthalmol. 2018; 32(1): 1–7. https://dx.doi.org/10.3341%2Fk....
79.
Flanagan KH, King R, Glaser DA. Botulinum toxin type a versus topical 20% aluminum chloride for the treatment of moderate to severe primary focal axillary hyperhidrosis. J Drugs Dermatol. 2008; 7(3): 221–227. PMID: 18380203.
80.
Hambleton P. Clostridium botulinum toxins: a general review of involvement in disease, structure, mode of action and preparation for clinical use. J Neurol. 1992; 239(1): 16–20. https://doi.org/10.1007/BF0083....
81.
Lowe NJ, Glaser DA, Eadie N, et al. North American Botox in Primary Axillary Hyperhidrosis Clinical Study Group. Botulinum toxin type A in the treatment of primary axillary hyperhidrosis: a 52-week multicenter double-blind, randomized, placebo-controlled study of efficacy and safety. J Am Acad Dermatol. 2007; 56(4): 604–611. https://doi.org/10.1016/j.jaad....
82.
Naumann M, Lowe NJ. Botulinum toxin type A in treatment of bilateral primary axillary hyperhidrosis: randomised, parallel group, double blind, placebo controlled trial. BMJ. 2001; 323: 596–599. https://doi.org/10.1136/bmj.32....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.