Online first
About the Journal
Current issue
Archive
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Office
Editorial Board
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Analysis of rs7895833 polymorphism of SIRT1 gene and its influence on the risk occurrence and progression of neurodegenerative disease, such as primary open-angle glaucoma in a Polish population
1
Department of Clinical Chemistry and Biochemistry, Medical University, Łódż, Poland
2
Department of Neurosurgery, Spine and Peripheral Nerve Surgery, Medical University, Łódż, Poland
3
Department of Gerontology, Geriatrics and Social Work, Jesuit University Ignatianum, Kraków, Poland
4
Laser Eye Microsurgery Center, Clinic of Jerzy Szaflik, Warsaw, Poland
5
Department of Ophthalmology, SPKSO Ophthalmic Hospital, Medical University, Warsaw, Poland
Corresponding author
Mateusz Siwak
Department of Clinical Chemistry and Biochemistry, Medical University of Lodz, Lodz, Poland, Al. T. Kościuszki 4, 90-419 Łódź, Poland
Department of Clinical Chemistry and Biochemistry, Medical University of Lodz, Lodz, Poland, Al. T. Kościuszki 4, 90-419 Łódź, Poland
J Pre Clin Clin Res. 2022;16(4):127-130
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
A neurodegenerative disease, which is primary open-angle glaucoma (POAG) through damage of the optic nerve, leads to irreversible loss of vision. Sirtuins are responsible for regulating the metabolism involved in brain aging and neurodegenerative disorders. Previous studies revealed that upregulation of SIRT1 has an important protective effect against various ocular diseases, such as cataract, retinal degeneration, optic neuritis and uveitis. Moreover, some experimental studies in animal models demonstrated a neuroprotective effect of SIRT1 against retinal and optic nerve damage. Therefore, the purpose of this study was to explore, for the first time, rs7895833 polymorphism of SIRT1 gene and its influence on the risk occurrence and progression of POAG in the Polish population.
Material and methods:
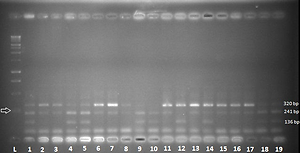
The study included 187 glaucoma patients and 171 controls.DNA was isolated from peripheral blood. Gene polymorphism was analyzed by restriction of the fragment length polymorphism-polymerase chain reaction (RFLP-PCR).
Results:
A statistically significant correlation was observed between the AG variant of the rs7895833 polymorphism of the SIRT1, and the occurrence of POAG. Moreover, a statistically significant correlation was observed between the rs7895833 polymorphism of the SIRT1, depending on the nerve fibre layer analyzer (GDx) (p = 0.034).
Conclusions:
Analysis of the rs7895833 polymorphism SIRT1 gene in the Polish population with POAG shows a higher prevalence of heterozygote A/G polymorphism than the control group, and the correlation between the nerve fibre layer analyzer (GDx) and SIRT1 gene polymorphism, which suggest that variant A / G polymorphism rs7895833 of the SIRT1 gene may have a protective effect on the occurrence of JPOK in the Polish population.
A neurodegenerative disease, which is primary open-angle glaucoma (POAG) through damage of the optic nerve, leads to irreversible loss of vision. Sirtuins are responsible for regulating the metabolism involved in brain aging and neurodegenerative disorders. Previous studies revealed that upregulation of SIRT1 has an important protective effect against various ocular diseases, such as cataract, retinal degeneration, optic neuritis and uveitis. Moreover, some experimental studies in animal models demonstrated a neuroprotective effect of SIRT1 against retinal and optic nerve damage. Therefore, the purpose of this study was to explore, for the first time, rs7895833 polymorphism of SIRT1 gene and its influence on the risk occurrence and progression of POAG in the Polish population.
Material and methods:
The study included 187 glaucoma patients and 171 controls.DNA was isolated from peripheral blood. Gene polymorphism was analyzed by restriction of the fragment length polymorphism-polymerase chain reaction (RFLP-PCR).
Results:
A statistically significant correlation was observed between the AG variant of the rs7895833 polymorphism of the SIRT1, and the occurrence of POAG. Moreover, a statistically significant correlation was observed between the rs7895833 polymorphism of the SIRT1, depending on the nerve fibre layer analyzer (GDx) (p = 0.034).
Conclusions:
Analysis of the rs7895833 polymorphism SIRT1 gene in the Polish population with POAG shows a higher prevalence of heterozygote A/G polymorphism than the control group, and the correlation between the nerve fibre layer analyzer (GDx) and SIRT1 gene polymorphism, which suggest that variant A / G polymorphism rs7895833 of the SIRT1 gene may have a protective effect on the occurrence of JPOK in the Polish population.
Siwak M, Maślankiewicz M, Nowak-Zduńczyk A, Filipe B, Wojtczak R, Radek M, Kucharska E, Szaflik J, Szaflik JP, Majsterek IJ. Analysis of rs7895833 polymorphism of SIRT1 gene and its influence on the risk occurrence and progression of neurodegenerative disease, such as primary open-angle glaucoma in a Polish population. J Pre-Clin Clin Res. 2022; 16(4):
127–130. doi: 10.26444/jpccr/157149
REFERENCES (32)
1.
Quigley H. Number of people with glaucoma worldwide. American Journal of Ophthalmology. 1996;122(3):460.
2.
Tham Y, Li X, Wong T, Quigley H, Aung T, Cheng C. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology. 2014;121(11):2081–2090.
3.
McKinnon S. The Cell and Molecular Biology of Glaucoma: Common Neurodegenerative Pathways and Relevance to Glaucoma. Investigative Opthalmology & Visual Science. 2012;53(5):2485.
4.
Danesh-Meyer H, Levin L. Glaucoma as a Neurodegenerative Disease. Journal of Neuro-Ophthalmology. 2015;35(Supplement 1):S22–S28.
5.
Anastasiou D, Krek W. SIRT1: Linking Adaptive Cellular Responses to Aging-Associated Changes in Organismal Physiology. Physiology. 2006;21(6):404–410.
6.
Carafa V, Nebbioso A, Altucci L. Sirtuins and Disease: The Road Ahead. Frontiers in Pharmacology. 2012;3.
7.
Donmez G. The neurobiology of sirtuins and their role in neurodegeneration. Trends in Pharmacological Sciences. 2012;33(9):494–501.
8.
Frye R. Phylogenetic Classification of Prokaryotic and Eukaryotic Sir2-like Proteins. Biochemical and Biophysical Research Communications. 2000;273(2):793–798.
9.
Mimura T, Kaji Y, Noma H, Funatsu H, Okamoto S. The role of SIRT1 in ocular aging. Experimental Eye Research. 2013;116:17–26.
10.
Kim D, Nguyen M, Dobbin M, Fischer A, Sananbenesi F, Rodgers J, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. The EMBO Journal. 2007;26(13):3169–3179.
11.
Corpas R, Revilla S, Ursulet S, Castro-Freire M, Kaliman P, Petegnief V, et al. SIRT1 Overexpression in Mouse Hippocampus Induces Cognitive Enhancement Through Proteostatic and Neurotrophic Mechanisms. Molecular Neurobiology. 2016;54(7):5604–5619.
12.
Zhang F, Wang S, Gan L, Vosler P, Gao Y, Zigmond M, et al. Protective effects and mechanisms of sirtuins in the nervous system. Progress in Neurobiology. 2011;95(3):373–395.
13.
Shindler K, Ventura E, Rex T, Elliott P, Rostami A. SIRT1 Activation Confers Neuroprotection in Experimental Optic Neuritis. Investigative Opthalmology & Visual Science. 2007;48(8):3602.
14.
Higashibata T, Wakai K, Naito M, Morita E, Hishida A, Hamajima N, et al. Effects of self-reported calorie restriction on correlations between SIRT1 polymorphisms and body mass index and long-term weight change. Gene. 2016;594(1):16–22.
15.
Kilic U, Gok O, Erenberk U, Dundaroz M, Torun E, Kucukardali Y, et al. A Remarkable Age-Related Increase in SIRT1 Protein Expression against Oxidative Stress in Elderly: SIRT1 Gene Variants and Longevity in Human. PLOS ONE. 2015;10(3):e0117954.
16.
Maszlag-Török R, Boros FA, Vécsei L, Klivényi P. Gene variants and expression changes of SIRT1 and SIRT6 in peripheral blood are associated with Parkinson’s disease. Sci Rep. 2021;11(1):10677. Published 2021 May 21. doi:10.1038/s41598-021-90059-z.
17.
Jęśko H, Wencel P, Strosznajder R, Strosznajder J. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochemical Research. 2016;42(3):876–890.
18.
Zhang T, Kraus W. SIRT1-dependent regulation of chromatin and transcription: Linking NAD+ metabolism and signaling to the control of cellular functions. Biochimica et Biophysica Acta (BBA) – Proteins and Proteomics. 2010;1804(8):1666–1675.
19.
Kovanen L, Donner K, Partonen T. SIRT1 Polymorphisms Associate with Seasonal Weight Variation. Depressive Disorders and Diastolic Blood Pressure in the General Population. PLOS ONE. 2015;10(10):e0141001.
20.
Kalemci S, Edgunlu T, Kara M, Turkcu U, Cetin E, Zeybek A. EXPERIMENTAL CARDIOVASCULAR AND LUNG RESEARCH Sirtuin gene polymorphisms are associated with chronic obstructive pulmonary disease in patients in Muğla province. Polish Journal of Cardio-Thoracic Surgery. 2014;3:306–310.
21.
Sabry D, Kaddafy S, Abdelaziz A, Nassar A, Rayan M, Sadek S, et al. Association of SIRT-1 Gene Polymorphism and Vitamin D Level in Egyptian Patients With Rheumatoid Arthritis. Journal of Clinical Medicine Research. 2018;10(3):189–195.
22.
Kilic U. Gok O. Bacaksiz A. Izmirli M. Elibol-Can B. Uysal O. SIRT1 Gene Polymorphisms Affect the Protein Expression in Cardiovascular Diseases. PLoS ONE. 2014;9(2):e90428.
23.
Ramkaran P, Phulukdaree A, Khan S, Moodley D, Chuturgoon A. Sirtuin 1 rs1467568 and rs7895833 in South African Indians with early-onset coronary artery disease. Cardiovascular Journal of Africa. 2016;27(4):213–217.
24.
Shimoyama Y, Mitsuda Y, Tsuruta Y, Suzuki K, Hamajima N, Niwa T. SIRTUIN 1 Gene Polymorphisms are Associated With Cholesterol Metabolism and Coronary Artery Calcification in Japanese Hemodialysis Patients. Journal of Renal Nutrition. 2012;22(1):114–119.
25.
Lee M, Choi S, Lee Y, Oh H. The Gender Association of the SIRT1 rs7895833 Polymorphism with Pediatric Obesity: A 3-Year Panel Study. Lifestyle Genomics. 2016;9(5–6):265–275.
26.
Kilic U, Gok O, Elibol-Can B, Ozgen I, Erenberk U, Uysal O, et al. SIRT1 gene variants are related to risk of childhood obesity. European Journal of Pediatrics. 2014;174(4):473–479.
27.
Shimoyama Y, Suzuki K, Hamajima N, Niwa T. Sirtuin 1 gene polymorphisms are associated with body fat and blood pressure in Japanese. Transl Res. 2011;157(6):339–347.
28.
Zillikens MC, van Meurs JB, Rivadeneira F, et al. SIRT1 genetic variation is related to BMI and risk of obesity. Diabetes. 2009;58(12):2828–2834. doi:10.2337/db09-0536.
29.
Botden IP, Zillikens MC, de Rooij SR, et al. Variants in the SIRT1 gene may affect diabetes risk in interaction with prenatal exposure to famine. Diabetes Care. 2012;35(2):424–426. doi:10.2337/dc11-1203.
30.
Rezaei Z, Kohan L, Yavarian M. Role of SIRT-1 rs7895833 polymorphism in susceptibility to polycystic ovary syndrome JSSU. 2017;24(11):861–867.
31.
Reus NJ, Lemij HG. Diagnostic accuracy of the GDx VCC for glaucoma. Ophthalmology. 2004;111(10):1860–1865. doi:10.1016/j.ophtha.2004.04.024.
32.
Kamal DS, Bunce C, Hitchings RA. Use of the GDx to detect differences in retinal nerve fibre layer thickness between normal. ocular hypertensive and early glaucomatous eyes. Eye (Lond). 2000;14(Pt 3A):367–370. doi:10.1038/eye.2000.90.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.