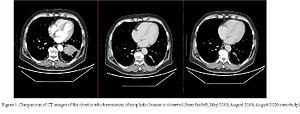
CASE REPORT
Efficacy of crizotinib therapy for a patient with non-small cell lung cancer with ALK gene
rearrangement – case report and review of current therapeutic options
1
Medical University, Lublin, Poland
2
Department of Pneumonology, Oncology and Allergology, Medical University, Lublin, Poland
J Pre Clin Clin Res. 2021;15(3):151-154
KEYWORDS
TOPICS
ABSTRACT
Introduction. Currently, nearly 23,000 cases of lung cancer are diagnosed in Poland annually, of which 5% are cases with rearrangements in the ALK gene. In recent years, tremendous progress has been made in understanding the genetic makeup of this type of cancer, which has enabled the use of new therapies, in particular, molecularly targeted drugs. Crizotinib is the first oral small molecule inhibitor of ALK, MET and ROS1 receptor tyrosine Kinases approved by the European Medicines Agency(EMA).
Case report. The paper presents the case of a 62-year-old patient diagnosed with non-small cell lung cancer with rearrangement in the ALK gene in stage IV of the disease. The patient was qualified for treatment with crizotinib under the B6 drug programme. Treatment started in May 2019. During treatment, assessment was made at 3 control points, where the first and second showed a partial response according to the RECIST 1.1 scale; in the next assessment, the response was maintained in the form of disease stabilization.
Bargieł JB, Cabaj J, Chmielewska I, Milanowski J. Efficacy of crizotinib therapy for a patient with non-small cell lung cancer with ALK gene rearrangement – case report and review of current therapeutic options. J Pre-Clin Clin Res. 2021; 15(3): 151–154. doi: 10.26444/jpccr/141598
REFERENCES (31)
1.
De Mello RA, Neves NM, Tadokoro H, et al. New Target Therapies in Advanced Non-Small Cell Lung Cancer: A Review of the Literature and Future Perspectives. J Clin Med. 2020; 9(11): 3543. https://doi.org/10.3390/jcm911....
2.
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2020; 71(3):209–249. https://doi.org/10.3322/caac.2....
3.
Tamura T, Kurishima K, Nakazawa K, et al. Specific organ metastases and survival in metastatic non-small-cell lung cancer. Mol Clin Oncol. 2014; 3: 217–221. https://doi.org/10.3892/mco.20....
4.
Herbst R, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018; 553: 446–454. https://doi.org/10.1038/nature....
5.
Vuong HG, Nguyen TQ, Nguyen HC, et al. Efficacy and Safety of Crizotinib in the Treatment of Advanced Non-Small-Cell Lung Cancer with ROS1 Rearrangement or MET Alteration: A Systematic Review and Meta-Analysis. Targ Oncol. 2020; 15: 589–598. https://doi.org/10.1007/s11523....
6.
Ang L, Chan CPY, Yau WP, et al. Association between family history of lung cancer and lung cancer risk: a systematic review and meta-analysis. Lung Cancer. 2020; 148: 129–137. https://doi.org/10.1016/j.lung....
7.
Kanwal M, Ding XJ, Cao Y. Familial risk for lung cancer. Oncol Lett. 2017; 13(2): 535–542. https://doi.org/10.3892/ol.201....
8.
Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014; 311(19): 1998–2006. https://doi.org/10.1001/jama.2....
9.
Fabbro D, Cowan-Jacob SW, Moebitz H, et al. Ten things you should know about protein kinases: IUPHAR Review 14. Br J Pharmacol. 2015; 172(11): 2675–700. https://doi.org/10.1111/bph.13....
10.
Bhullar KS, Lagarón NO, McGowan EM, et al. Kinase-targeted cancer therapies: progress, challenges and future directions. Mol Cancer. 2018; 19;17(1): 48. https://doi.org/10.1186/s12943....
11.
Shah DR, Shah RR, Morganroth J, et al. Tyrosine kinase inhibitors: their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013; 36(6): 413–26. https://doi.org/10.1007/s40264....
12.
Shaw AT, Riely GJ, Bang YJ, et al. Crizotinib in ROS1-rearranged advanced non-small-cell lung cancer (NSCLC): updated results, including overall survival, from PROFILE 1001. Ann Oncol. 2019; 30(7): 1121–1126. https://doi.org/10.1093/annonc....
13.
Hoang T, Myung S-K, Pham TT, et al. Efficacy of Crizotinib, Ceritinib, and Alectinib in ALK-Positive Non-Small Cell Lung Cancer Treatment: A Meta-Analysis of Clinical Trials. Cancers. 2020; 12(3): 526. https://doi.org/10.3390/cancer....
14.
Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013; 368(25): 2385–94. https://doi.org/10.1056/NEJMoa....
15.
Peters S, Camidge DR, Shaw AT, et al. ALEX Trial Investigators. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2017; 377(9): 829–838. https://doi.org/10.1056/NEJMoa....
16.
Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus Crizotinib in ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2018; 379(21): 2027–2039. https://doi.org/10.1056/NEJMoa....
17.
Selvaggi G, Wakelee HA, Mok T, et al. Phase III randomized study of ensartinib vs crizotinib in anaplastic lymphoma kinase (ALK) positive NSCLC patients: EXALT3. Presented at the International Association for the Study of Lung Cancer World Conference on Lung Cancer, Singapore, August 8, 2020.
18.
Camidge DR, Dziadziuszko R, Peters S, et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-positive advanced non-small cell lung cancer in the global Phase III ALEX Study. J Thorac Oncol. 2019; 14: 1233–1243. https://doi.org/10.1016/j.jtho....
19.
Camidge DR, Kim HR, Ahn M-J, et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the Phase III ALTA-1L trial. J Clin Oncol. 2020; 1; 38(31): 3592–3603. https://doi.org/10.1200/jco.20....
20.
Płużański A. Kryteria oceny odpowiedzi na leczenie RECEIST 1.1. Nowotwory. J Oncol. 2014; 64(4): 331–335. https://doi.org/10.5603/NJO.20....
21.
Novello S, Mazières J, Oh I-J, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018; 29(6): 1409–1416. http://doi: 10.1093/annonc/mdy121.
22.
Shaw AT, Bauer TM, de Marinis F, et al. CROWN Trial Investigators. First-Line Lorlatinib or Crizotinib in Advanced ALK-Positive Lung Cancer. N Engl J Med. 2020; 383(21): 2018–2029. https://doi.org/10.1056/NEJMoa....
23.
Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 inhibitor, overcomes resistance to first and second generation ALK inhibitors in preclinical models. Cancer Cell. 2015; 28: 70–81. https://doi.org/10.1016/j.ccel....
24.
Horn L, Whisenant JG, Wakelee H, et al. Monitoring therapeutic response and resistance: analysis of circulating tumor DNA in patients with ALK+ lung cancer. J Thorac Oncol. 2019; 14: 1901–1911. https://doi.org/10.1016/j.jtho....
25.
Johnson TW, Richardson PF, Bailey S, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(metheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014; 57: 4720–4744. https://doi.org/10.1021/jm5002....
26.
Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK-rearranged lung cancer. Cancer Discov. 2016; 6: 1118–1133. https://doi.org/10.1158/2159-8....
27.
Solomon B, Beauer TM, Marinis De F, et al. Lorlatinib vs Crizotinib in the First-line Treatment of Patients (pts) with Advanced ALK-Positive Non-Small Cell Lung Cancer (NSCLC): Results of the Phase 3 CROWN Study. Ann Oncology. 2020; 31(4): 1180–1181. https://doi.org/10.1016/j.anno....
28.
Lin M.H, Pan X, Hou P, et al. Real-world treatment duration in ALK-positive non-small-cell lung cancer patients receiving brigatinib through the early access program. Future Oncol. 2020; 16(15): 1031–1041. http:// doi: 10.2217/fon-2019-0849.
29.
Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib Versus Crizotinib in Advanced ALK Inhibitor-Naive ALK-Positive Non-Small Cell Lung Cancer: Second Interim Analysis of the Phase III ALTA-1L Trial. J Clin Oncol. 2020; 1; 38(31): 3592–3603. https://doi: 10.1200/JCO.20.00505.
30.
Shaw AT, Kim TM, Crinò L, et al. Ceritinib versus chemotherapy in patients with ALK-rearranged non-small-cell lung cancer previously given chemo-therapy and crizotinib (ASCEND-5): a randomized, controlled, open-label, phase 3 trial. Lancet Oncol. 2017; 18(7): 874–886. https://doi.org/10.1016/S1470-....
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.